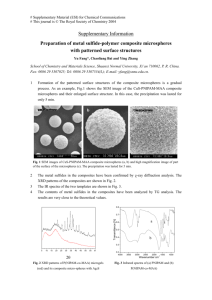
The vertical deposition self-assembly process and the formation
advertisement

The vertical deposition self-assembly process and the formation
mechanism of poly(styrene-co-methacrylic acid) photonic
crystals on polyester fabrics
Guojin Liua, Lan Zhoua*, Qinguo Fana,b, Liqin Chaia and Jianzhong Shaoa*
a
Engineering Research Center for Eco-Dyeing and Finishing of Textiles, Ministry of Education,
Zhejiang Sci-Tech University, Hangzhou 310018, China
b
Department of Bioengineering, University of Massachusetts Dartmouth, North Dartmouth, MA
02747, USA
“*”denotes corresponding author
Fax: 86-571-86843666; Tel: 86-571-86843610;
E-mail: lan_zhou330@163.com; jshao@zstu.edu.cn
1
Electronic supplementary
1. Synthesis of P(St-MAA) colloidal microspheres
Main monomer
Functional monomer
nSt+mMAA APS
Initiator
P(St-MAA)
Polymer
Figure 1. Schematic diagram of synthesis of P(St-MAA) colloidal microspheres.
Batches of monodisperse P(St-MAA) microspheres with diameters in the range of 200–400 nm
were prepared by soap-free emulsion copolymerization. The size of the P(St-MAA) microspheres
produced in this method is highly dependent on the different concentrations of St, MAA, and APS.
Syntheses were carried out in a fournecked round-bottom flask equipped with an inlet of nitrogen
gas, a reflux condenser, thermometer, and a mechanical stirrer. With one sample as an example,
the procedure used was outlined as follows: 20 g St, 3 g MAA, and 195 g H2O were added to the
four-necked round-bottom flask. Nitrogen gas was then slowly bubbled through the resulting
two-phase system and vigorous mechanical stirring of the mixture commenced. When the mixture
was heated to 70 °C, 0.1 g APS dissolved in 5 g H2O was introduced into the reactor. The reaction
was maintained at 70 °C for 8 h. The whole reaction was carried out in nitrogen atmosphere with
mechanical stirring at around 350 rpm. The resulting colloidal suspension of P(St-MAA)
microspheres was then filtered through a glass wool plug to remove any large agglomerates and
then stored in PET plastic bottles for later use. When the parameters of the polymerization were
changed, P(St-MAA) microspheres of different diameters can be prepared.
2. Characterization of P(St-MAA) colloidal microspheres
Figure 2(a) presents a typical TEM photograph of the P(St-MAA) microspheres. It could be
2
seen that the P(St-MAA) microspheres with the core-shell architecture were spherical in shape and
monodisperse in size. In each latex microsphere, the darkly stained regions were surrounded by
the lighter thin domains and the thickness of the shell part was about 20-30 nm (see insert to
Figure 2 (a) ). The hydrophilicity of the comonomer was the main polymerization parameter that
affected the latex microsphere morphology, i.e., the outermost layer of the latex microsphere was
different from its interior, being enriched with polar groups. The scheme of the P(St-MAA)
colloidal microsphere was shown in Figure 2 (b). Based on the polarity and hydrophilicity of the
comonomers (St and MAA), as well the relative polymers: PS domains were located on the core
of the latex microspheres, and there was a thin shell rich in PMAA covers the PS core polymer
with carboxyl groups anchored upon the surface of latex microspheres, which favored the
formation of hydrogen bonds among latex microspheres.
a
b
100nm
200nm
Figure 2 (a) Typical TEM image of core-shell P(St-MAA) microspheres with a diameter of 210
nm and PDI value smaller than 0.08; (b) Scheme of the structure of the as-prepared latex
microsphere.
In order to confirm the above analysis, X-ray photoelectron spectroscopy (XPS) was performed
to clarify the surface elemental composition of P(St-MAA) microsphere and its core-shell
structure in Figure 3. The full-scan spectrum in Figure 3 (a) shows that the surface elements of the
3
prepared P(St-MAA) microspheres were carbon and oxygen (hydrogen cannot be detected by
XPS). The C1s spectrum in Figure 3 (b) clearly shows the presence of two types of carbons at
binding energies of 284.8 and 289.3 eV, respectively. The former is attributed to the carbons in the
C-C and C-H linkages, while the latter is attributed to the carbons in the carboxyl groups, i.e.,
-COOH group. A typical O1s spectrum shown in Figure 3 (c) is mainly composed of two peaks at
532.6 and 533.8 eV, which are attributed to the two oxygen atoms in –COOH group, respectively.
Therefore, the XPS results verified the core-shell structure scheme of P(St-MAA) microspheres in
Figure 2 (b).
180000
80000
a
160000
C1s
b
284.8
C1s
70000
60000
120000
Counts / s
Counts / s
140000
100000
80000
O1s
60000
50000
40000
30000
20000
40000
289.3
10000
20000
0
0
0
200
400
600
800
1000
1200
280
282
284
286
288
290
292
Binding Energy (ev)
Binding Energy (ev)
30000
c
O1s
25000
Counts / s
532.6
533.8
20000
15000
10000
5000
528
530
532
534
536
538
540
Binding Energy (ev)
Figure 3 XPS spectra of P(St- MAA) microspheres. (a) full-scan; (b) C1s spectrum; (c) O1s
spectrum.
3. Three-dimensional face-centered cubic structure of P(St-MAA) photonic
crystals on textile fabrics
4
a
b
[10
0]
{100}
1]
[11
{111}
Figure 4 (a) The top-view of colloidal crystals on polyester fabric (×10000); (b) The different
crystalline planes of colloidal crystals on polyester fabric (×10000).
The ordered arrangement of the prepared P(St-MAA) photonic crystals was regarded as a
three-dimensional face-centered cubic (fcc) structure through the self-assembly of vertical
deposition, as shown in Fig. 4. The top-view of P(St-MAA) colloidal crystals on polyester fabric
is shown in Fig. 4(a). It is presented that the P (St-MAA) colloidal microspheres are packed in a
close-packed arrangement, with each microsphere neighbouring six others in a layer,
corresponding to (111) plane of fcc or (001) plane of hcp. Fig. 4(b) shows the cross section of
P(St-MAA) photonic crystal structure on polyester fabrics. It is observed that colloidal
microspheres are arranged closely and orderly over the entire substrate area, especially, square and
hexagon arrangements of P(St-MAA) microspheres are obviously shown, respectively. However, a
rectangular alignment with one microsphere in the center and four spheres at the corners,
corresponding to hcp stacking, is never found in this investigation, which meant that the resulting
close-packed structure of P(St-MAA) microspheres was not compatible with the hcp packing.
Actually, a square arrangement in Fig. 4(b) corresponds to {100} type plane of fcc structure, while
a hexagon arrangement corresponds to {111} type plane in fcc structure, which can directly
5
confirm the three-dimensional fcc structure of the resulting P(St-MAA) photonic crystals on
polyester fabrics in our work.
6