Inclusion/Exclusion Criteria
advertisement

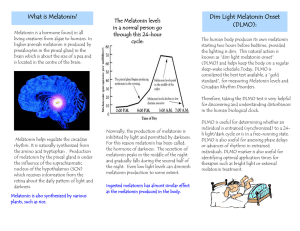
STAFF INFORMATION SHEET: MY NAP Trial: Melatonin in Youth N-of-1 trials in a stimulant-treated ADHD population Investigators Dr Jane Nikles, School of Medicine, The University of Queensland Prof Geoff Mitchell, School of Medicine, The University of Queensland Dr Hugh Senior, School of Medicine, The University of Queensland A/Prof Honey Heussler, Lady Cilento Children’s Hospital What is the MY NAP trial? This trial is a comparison of a series of randomised, double-blind, placebo-controlled, multi-centre, n-of-1 trials with a randomised controlled trial. The objectives are: 1. 2. To determine the efficacy of melatonin in shortening sleep latency times in children with ADHD treated with stimulants. To determine whether n-of-1 trials provide similar estimate of treatment effect with less uncertainty than a parallel group RCT. Who is suitable for the MY NAP trial? 1. 2. 3. 4. 5. 6. Children and adolescents between 6 and 17 years; Diagnosis of ADHD according to DSM- IV or V criteria On a stable dose of stimulant (e.g., Ritalin®, Ritalin LA, Concerta®) for at least 1 month prior to the study. The dosage will need to remain constant during the study for the data to be included in analysis; Sleep Onset Latency of 45 min, 3 nights/week, for 1 month as confirmed by parent/guardian; If previously on melatonin, have ceased it at least two weeks previously; Informed consent by parent/guardian and by child (if 12 years and over). Continued: University of Queensland School of Medicine Building 28, Salisbury Road Tel: 07 3381 1597 Fax: 07 3381 1356 Email: mynap@uq.edu.au Mater Medical Research Institute Aubigny Place, Mater Hospital Raymond Terrace Ipswich Q 4305 South Brisbane Q 4101 Who is NOT suitable for the MY NAP trial? 1. Children with co-morbid psychiatric/neurological diagnoses that may affect sleep, including: -autism/pervasive development disorder -brain injury -cerebral palsy -uncontrolled major depression -migraines -posttraumatic stress disorder -psychosis or schizophrenia - seizure disorder (i.e. seizure in the last 12 months) 2. Children with any of the following disorders of sleep: -Untreated Obstructive Sleep Apnea -Sleep Related Breathing Disorder (any form of trouble during sleep associated with breathing, such as need for oxygen, underlying lung disease outside of stable asthma) -Untreated narcolepsy -Sleep related movement disorders (head banging or body rocking that results in insomnia) -Parasomnias (current, regular sleep walking or night terrors) -Adjustment insomnia (acute – related to hospitalization) -Insomnia due to drug use, or mental health issue -Secondary enuresis 3. Known allergy or hypersensitivity to melatonin or other study drug ingredients; Mannitol, Dextrose, Cellulose, Crospovidone, Calcium Carbonate, Xylitol, Dicalcium Phosphate, Vegetable Stearic Acid, Vegetable Magnesium Stearate, Silica; Children on immunosuppressive drugs, blood pressure drugs, SSRIs or anticoagulant drugs; Children not on regular sedatives or hypnotics whose parents do not agree not to commence these treatments regularly during the course of the trial. Parents of children on sedatives or hypnotics who do not agree not to alter the daily dose of these for the duration of the trial. Patients with active or uncontrolled hormonal disorders, or diabetes, or active liver disease, or abnormal kidney function or untreated kidney disease, or any blood clotting disorders; Participants who disagree to not driving or operating heavy machinery within 8 hours of ingestion of study medication; Breastfeeding or pregnant women; Girls 12 years and above who are menstruating and sexually active; Children whose parent/primary caregiver does not understand English, or have a phone. 4. 5. 6. 7. 8. 9. 10. 11. MyNAP Staff Information Sheet V2 April 2015 2 What will be required of the patient and their parent/guardian? In the first phase, the parent/s would be asked to initially complete some questionnaires and be given information about healthy sleeping habits. Their child’s sleeping activity would be monitored with an actigraph watch for two weeks. The parents would also be asked to keep a daily sleep diary during this period. At the end of the fortnight, data from the sleep diary would be analysed. If sleep onset latency is still 45 min, 3 nights/week the child would be eligible for the trial. During the trial, the child would be given the medication (either melatonin or a placebo) for 1 week blocks, for six weeks. Throughout this period, the parent/s would be asked to keep a daily sleep diary and complete more questionnaires. Their child will wear an actigraph watch during the six weeks’ trial period, to assist in measuring their sleep activity. Frequently asked questions 1. Will patient information be kept confidential? All patient information collected during the project will be kept completely confidential. Any written or published documents regarding the trial will not contain information identifying participants. 2. Are there any possible risks? Short-term (6 weeks) supplementation of high doses (up to 100 mg/day) of exogenous melatonin in healthy young subjects is not associated with serious adverse effects. Minor effects reported with repeated short-term melatonin use were nausea, headache, dizziness, and drowsiness (9.5% pooled subjects); not significantly different to placebo (5% pooled subjects). Safety for long-term paediatric use of melatonin (>6 weeks of administration) has been examined by investigators who followed 105 children aged 6 to 12 years for a mean period of 3.7 years (37). Melatonin use was not associated with serious harm. Adverse events reported included: dizziness (4.3%), bedwetting (3.2%): likely to be secondary to sleeping deeply, sleep maintenance insomnia (3.2%) likely to be due to phase shifting too far, nausea (2.1%), skin pigment changes (2.1%), nightmares (2.1%), visual disturbances (2.1%), excessive morning sedation (2.1%) giving medication too late, constipation (1.1%), profuse perspiration (1.1%), decreased mood (1.1%), daytime laziness (1.1%), and change in behaviour (1.1%). Melatonin use in children has become widespread. It is imperative that its safety and efficacy are evaluated systematically. We conclude that the risk of conducting a short-term crossover study in this population is minimal. There are reports of other side effects, however these are usually related to timing of medication and settle on ceasing the medication. There have been theoretical concerns about alterations in pubertal hormonal status however this has not been able to be proven in clinical studies. Low melatonin levels have been noted in girls with early puberty. The effect of supplementation is unknown but theoretically may delay onset of puberty. Other concerns have arisen about epileptogenicity of melatonin in children with neurodevelopmental disorders in a small case series. Sleep is a risk time for seizures and it is hard to determine what the effect in this study was. Other studies have not confirmed any issues. In all case reports the seizures ceased once the melatonin was ceased. Clinically it is used in children with epilepsy with no anecdotal evidence that it worsens seizure threshold. There is very little evidence in children and the study cited is probably the most comprehensive. Continued: MyNAP Staff Information Sheet V2 April 2015 3 3. Are there any incentives to the patients? The only incentive for this trial is the determination of Melatonin feasibility in managing sleep and sleep onset latency, for the patient involved. No other incentives exist in this trial. 4. Sponsors of the service The organisation sponsoring this trial is The University of Queensland, and the study is funded by the NHMRC. Ethics Approval This study has been cleared by: - Human medical research ethics committee of the University of Queensland (Ethics approval number 2012000999) - Mater Health Services Human Research Ethics Committee (HREC/14/MHS/28/AM01) - Children's Health Queensland Hospital and Health Service - Human Research Ethics Committee - Human Research Ethics Board of the University of Alberta, Canada If you would like to speak to an officer not involved in the study, you can contact the UQ Ethics Officer on 3365 3924, the Mater HREC Co-ordinator on 3163 1585, the Children’s Health Queensland Hospital and Health Service Research Governance Officer on 3636 4445, or the West Moreton Health Service District HREC administrator on 3271 8656. For Further Information Please contact the Research Project Manager with any queries about patient eligibility or the trial: Name: Phone: Email: Jane Nikles 07 3381 1597 mynap@uq.edu.au MyNAP Staff Information Sheet V2 April 2015 4