HF Manual
advertisement

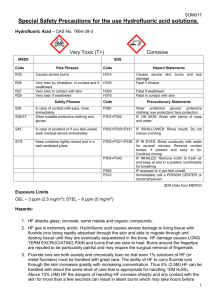
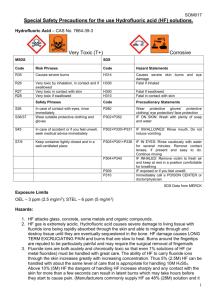
School of Chemistry SAFETY MANUAL FOR THE HANDLING OF HYDROFLUORIC ACID SOLUTIONS No researcher should use solutions of hydrofluoric acid unless they have read and understood these notes and have agreed to abide by the recommendations, protocols and prohibitions contained herein. Your research supervisor must be aware that you are engaged in such work, and other occupants of the laboratory should also be informed. The protocols outlined in this manual constitute a COSHH assessment. 2013 Hydrofluoric Acid Hydrofluoric acid – a solution of hydrogen fluoride in water – is an extremely corrosive, very toxic and dangerous reagent. Exposure to hydrofluoric acid can cause severe tissue damage and deaths have been reported from exposure of relatively small areas of tissue to concentrated (>50%) solutions. Hydrofluoric acid is absorbed very quickly, but symptoms may be delayed at lower concentrations. The following safety precautions should be followed irrespective of the concentration of hydrofluoric acid that is used. Specialist medical treatment is required for anyone who is exposed. 1. Toxicity and Hazards Hydrofluoric acid is extremely poisonous and corrosive and both the vapour and liquid are hazardous. Exposure of tissue to hydrofluoric acid causes severe and painful burns which may not be immediately painful or visible because of the anesthetic properties of less concentrated solutions. The liquid and vapour can burn the skin, eyes and respiratory tract. Ingestion of the liquid or inhalation of the vapour can be fatal. Exposure can also lead to severe bone damage. Long term exposure can lead to damage of the liver and kidneys. Skin Hydrofluoric acid vapour and liquid cause severe burns may not be immediately painful or visible. Hydrofluoric acid penetrates the skin rapidly and damages underlying tissues. Large or multiple burns can cause hypocalcemia (calcium depletion) and other toxic effects, which can be fatal. Prolonged contact with very dilute solutions of hydrofluoric acid can also result in burns – this can occur when a victim is unaware that he/she has been exposed. Eyes Both vapour and liquid can cause severe irritation and corneal burns. Exposure of the eyes to HF can result in blindness. Inhalation Mild exposure can irritate the nose, throat and respiratory system and a shortness of breath. The onset of symptoms can be delayed for several hours. Severe exposure results in burns to the nose, throat and lungs and can result in lung 1 inflammation and pulmonary oedema (fluid in the lungs). Exposure can cause hypocalcemia (calcium depletion) and other effects which can be fatal. Ingestion Can cause severe burns to the mouth, throat and stomach, which may be fatal. Even small amounts of relatively dilute solutions can result in fatal hypocalcemia (calcium depletion) and systemic poisoning is likely to occur if immediate medical treatment is not given. Delayed effects The onset of symptoms may be delayed following exposure to dilute solutions of hydrofluoric acid or the vapours of these solutions. The expected delays are as follows: 50% concentration – immediate onset 20–50% concentration – 1 to 8 hours <20% concentration – up to 24 hours Delayed symptoms may include pain, redness of the skin and tissue destruction. Prolonged exposure can lead to long term adverse changes to bone and joints (fluorosis). Hydrofluoric acid can react with certain metals to generate hydrogen gas which is flammable and potentially explosive. It also attacks glass and other silicon-containing compounds and materials. Hydrofluoric acid reacts with silica to produce the hazardous and colourless gas silicon tetrafluoride. 2. Handling and Storage Hydrofluoric acid solutions should be kept in sealed polyethylene containers. The containers should be stored in a cool dry place well away from reactive chemicals. Care should be taken to avoid damaging the container and secondary containment should be used wherever possible. 3. Working with Hydrofluoric Acid Solutions Glass flasks will become etched on exposure to a solution of hydrofluoric acid, even when the solution is relatively dilute. For prolonged reactions and those involving more concentration solutions of hydrofluoric acid, a polyethylene reaction vessel may be an appropriate choice. Solutions of hydrofluoric acid must only be handled in a fume cupboard and never on the 2 open bench. When handling hydrofluoric acid solutions, two pairs of gloves should be worn – a pair of disposable gloves should be worn underneath more robust heavy-duty gloves (PVC or neoprene). Before commencing work, make absolutely sure that both pairs of gloves are in good condition and do not contain any tears or holes. If a large volume of the hydrofluoric acid solution is to be used, safety glasses and a full face-shield must be worn. Make sure that a tube of calcium gluconate HF antidote gel is to hand and that it is ‘in date’. Be very careful to avoid any inadvertent contamination of the skin or clothing when transferring hydrofluoric acid solutions. Make sure that legs and feet are completely protected in case of splashes. 4. Spillages Spills should be diluted with water (to concentration <50%) and then neutralised with sodium hydroxide, sodium carbonate, calcium hydroxide or calcium carbonate. 5. Health Effects and First Aid Skin exposure Remove victim to an uncontaminated area. Decontamination should be performed immediately even if the solution is dilute and exposure is limited. Flush exposed area with copious amounts of cold water for a period of 15 minutes under an emergency shower or in the sink. Remove all contaminated clothing whilst flushing with water – make sure those who are assisting are wearing gloves. Apply calcium gluconate gel to the affected area with a gloved hand and continue to massage gel into the burn area for a period of 20 minutes. If a whitening of the skin occurs wash off the gel and apply fresh calcium gluconate gel. Call for emergency medical assistance or transfer to hospital emergency department. Eye exposure Contact lenses must not be worn when handling HF. 3 Flush eye(s) with copious amounts of cold water for a period of 30 minutes under an emergency shower or in the sink – make sure those who are assisting are wearing gloves. Keep eyelids apart and away from the eyeball when irrigating the eye. If a single eye is affected, make sure that contaminated water is not flushed into the other eye. Call for emergency medical assistance or transfer to hospital emergency department. If possible, continue irrigation of the eye(s) during transportation to hospital. Inhalation The exposure threshold is 0.04 ppm and exposure levels above 20 ppm are considered lifethreatening and will result in severe damage to the lungs and other organs. HF has a strong irritating odour, but an absence of a smell does not indicate that you have not been exposed. Move to location where the victim can breathe fresh air. Keep victim lying down and warm. Call for emergency medical assistance or transfer to hospital emergency department. Ingestion Rinse the victim’s mouth with cold water, but under no circumstances induce vomiting. If the victim is conscious give him/her copious amounts of water to dilute the acid. Call for emergency medical assistance or transfer to hospital emergency department. It should be noted that even if the victim is displaying only mild symptoms of exposure, he/she must seek professional medical attention because symptoms and the true severity of exposure may only become apparent several hours after exposure. 4