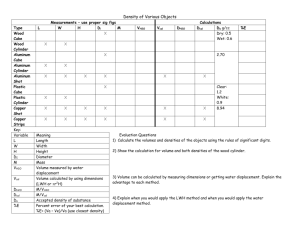
PRESENTATION
advertisement

INDEX 3 Presentation 4 The CNR Center: A short story 5 The Collaborative Relationship between the Department of Biology and the CNR Center 6 CNR and University personnel 7 Main Research Lines: 1) Metals and the Brain: 7 a. Aluminum as a Neurotoxic Agent: Relevance of the Metal Speciation in Human and Experimental Toxicology. 10 b. Metallothioneins, Aging and Neurodegenerative Diseases 12 c. Aluminum and the Biophysical State of Cell Membrane. 14 2) Oxidative Stress induced by Photodynamic Effect on the Structure and Function of cell and Subcellular Organelles 17 3) Sovramolecular Organization, Functional Regulation and bioinorganic Properties of Invertebrate Oxygen Carriers 23 4)Photosensitizing and Phototherapeutic Properties of Porphyrins and their analogues 29 Publications 51 Communications 63 Reviews and Books Chapters 64 Books 65 International Projects 66 National Projects 66 International Collaborations 66 National Collaborations 67 International and National Conferences 67 Theses 70 PhD Theses 71 Acknowledgments 2 PRESENTATION The Italian National Research Council (CNR) is currently in the throes of a major reorganization that will shortly see its approximately 300 organizations (Institutes and Centers) rearranged into 100 National Institutes through the merging or suppression of the original structures. The CNR Center for Research in Biochemistry and the Physiology of Metalloproteins, situated at the Biology Department of the University of Padua, has already been reorganized. In fact, by decree of the President of the CNR on 18.4.2001, it has become a separate section of the Biomedical Technologies Institute (ITB) of Milan. The ITB also comprises sections dealing with Epidemiology, Analytical Biochemistry, Bio-informatics and Cell Pathology. In its 30 years of activity, the Metalloproteins Center in Padua has naturally seen its research programs evolve progressively from studies on hemocyanins alone to research projects on the Biochemistry and Biophysics of Cuproproteins, Neurobiology, and Photobiology. These three decades have witnessed an important, close scientific cooperation with the University of Padua, and the Biology Department in particular, based on a convention for pooling ideas, people and equipment. Moreover, in its 30 years of life, the Center has had numerous Italian and international visiting scholars as its guests on scientific cooperation schemes, as well as students preparing their graduate and postgraduate dissertations, or in postgraduate training, professional placements, etc., who have all contributed something of their own resources. It has been gratifying to see that the scientific productivity of the Metalloproteins Center has been one of the most significant of all the research centers of the Italian National Research Council. Finally, over the years, the Center has further developed its scientific heritage through important forms of cooperation with other national and international research organizations, exchanging foreign visitors, organizing national and international conferences, coping with an extensive publishing activity and more besides. We are therefore convinced that we have made good use of the taxpayers' funds in the general interest of the community. Though the future horizons emerging before us are still not entirely clear, I sincerely hope that - with the reformed CNR – the cooperation between our section and the University of Padua will continue well into the future, in our mutual interest and to the benefit of the scientific community. Paolo Zatta Section Manager 3 THE CNR CENTER: A SHORT STORY At the end of August 1966, before definitively leaving the Naples Zoological Station where I had held the position of Head of the Physiological Department since 1954, I organised a convention on “Biochemistry and Physiology of Hemocyanins”, which represented the culmination of the research, funded by yearly contributions from the NIH (National Institute of Health), which I had been conducting at that Institute for a number of years. The convention was held at the Zoological Station from August 30 to September 1, 1966(1). It was attended by about thirty Italian and foreign researchers who worked in the field of hemocyanins and metalloproteins. These included E.F.G. van Bruggen, R. Lontre, A.C. Redfield, J.R. Redmond, E. Antonimi, A. Ehrenberg, C.B. Malmstrom, G. Morpurgo, K.E. van Holde, R.J.P. Williams, B.L. Vallee and J. Wyman. That convention marked the beginning of a series of workshops, held every two to three years at the principal European centres where research on hemocyanins was being conducted. The last workshop was held in Roscoff, France, in the year 2000. In 1967, we were informed that the NIH and the other American research centres intended to discontinue the financial support accorded for all the research programs. In practice, this meant the sudden termination of all the biological and biomedical research being done in Italy. All the interested parties decided to meet in Milan, where we agreed to explain the situation and appeal to the Italian government for assistance. As a result, the National Research Council (CNR) took over the various research programs and reorganised them as Research Centres based at various Universities. This is how the CNR Centre for the Study of the Biochemistry and Physiology of Hemocyanins and other Metalloproteins was established at the University of Padua at the end of 1969. The research activities conducted by the Centre can be inferred from the list of scientific studies published to date. F.Ghiretti Professor Emeritus of General Physiology 1. Physiology and Biochemistry of Hemocyanins. F. Ghiretti Editor. Academic Press, 1968. 4 THE COLLABORATIVE RELATIONSHIP BETWEEN THE DEPARTMENT OF BIOLOGY AND THE CNR CENTER When in 1971 I participated in the first competitive examination announced for a position as researcher at the newly-established the Center for the Study the Physiology and Biochemistry of Hemocyanins, of the National Research Council (CNR), I certainly never imagined that I would find myself in the position of Head of the hosting Department, presenting the work the Center has carried out over 30 years of research. Prof. Francesco Ghiretti, who the Science Faculty had asked to take the chair of General Physiology, arrived in Padua with a research group that was studying proteins using biochemical methods. The event spurred the interest and expectations of staff in the then Institute of Zoology, Comparative Anatomy and Genetics. Those who were using completely different research methods, for years unsuccessfully tackling specific issues, nurtured the hope that collaboration with the new group could help resolve their own problems. More in general, it was thought that through collaboration, the new methodology's contribution could stimulate more up-to-date and competitive research orientations. Seminars and meetings were held to identify possible forms of interaction, but collaboration was difficult, as research fields were very diverse and the issues confronted in the Center were quite particular, precisely because they were linked to the specific goal of the institution itself, Hemocyanins. As the years passed, the Center gained new personnel and collaborated to some extent with university professors, whose backgrounds were nevertheless primarily in chemistry. Gradually, it began to grow independent and almost separate from the hosting Institute, while it cultivated collaboration and comparison with outside researchers who were interested in the same research issues. Things did not change much even when it became necessary to broaden the field of research from Hemocyanins to other copper proteins, and later metal proteins. In the meantime, the Institute became the Department of Biology with the merging of the institutes of Botany and Anthropology, the sphere of research diversified greatly, and the advent of new technologies in the field of molecular biology and biotechnology gave it a different face even from its recent past in biological research. Over the past few years, the CNR has also undergone a profound transformation that has substantially changed the body's organization and use of resources, leading, among other things, to the closing of study centers as autonomous structures and merging them into the Institutes of which they have become sections. We are thus witnessing a significant innovative ferment that involves both the university and the CNR, creating conditions in which the personnel of the new bodies can engage in profitable scientific collaboration, which has actually developed in the recent past, but only sporadically. The solicitude with which the reseachers of the CNR Section has initiated relationships with other members of the Institute of Biomedical Technologies into which they have been merged and with university researchers who have been collaborating with this Institute for some time, as well as the diligence demonstrated in soliciting research groups from the Department to interact with their themes, are cause for hope that a CNR structure within the Department of Biology is not just a coincidence, but rather an opportunity for organic, continuous and advantageous collaboration. Arnaldo Cassini Prof. of General Physiology Head of Biology Department of the University of Padua 5 CNR and University personnel Directors Prof. Francesco Giretti Prof. Anna Giretti Magaldi Prof. Benedetto Salvato Dr Paolo Zatta 1970-1990 1990-1996 1997-2001 2001- CNR Personnel Researchers Dr Arnaldo Cassini Dr Fernanda Ricchelli Dr Laura Tallandini Dr Paolo Zatta 1970-1974 19751970-1974 1976- CNR Staff Mr Ennio Carbone Mr Antonio Cervellin Mr Daniele Cervellin Mr Sivano Gobbo MrGiampaolo Rocco Mr Giuseppe Tognon F.A. O.T. CTER O.T. CTER CTER 197719731971197819691974- University personnel Prof. Vincenzo Albergoni Prof. Mariano Beltramini Prof. Giulio Jori Prof. Anna Ghiretti Magaldi Prof. Benedetto Salvato 1970-1979 199119771970-1996 1970- University Staff Mr Renato Carbone Mr Manuela Nicoletti 1970-1988 1982-1987 Members of the Scientific Committee Prof. Lucia Banci Department of Chemistry, University of Firenze Prof. Umberto Belluco Department of Industrial Chemistry,University of Padova Prof. Maurizio Brunori Department of Biochemistry, University of Roma Prof. Luigi Casella Department of Chemistry, University of Pavia Prof. Heinz Decker Institut für Molekular Biophysic, University of Mainz, Germany Prof. Giorgio Giacometti Department of Biology, University of Padova Prof. Marino Nicolini Department of Pharmacological Sciences, University of Padova Prof. Ferdinando Palmieri Department of Biology and Pharmacology, University of Bari Prof. Giovanni Porcellati Department of Biochemistry, University of Perugia Prof. R.J.P. Williams Department of Inorganic Chemistry, University of Oxford, UK 6 Main Research Lines 1 METALS AND THE BRAIN a. ALUMINUM AS A NEUROTOXIC AGENT: RELEVANCE OF THE METAL SPECIATION IN HUMAN AND EXPERIMENTAL TOXICOLOGY. In spite of the natural abundance of Al(III) in the biosphere, no useful biological function of this metal ion has been discovered so far. On the contrary, Al(III) is now well recognized as a neurotoxic metal center. The first experimental toxicological study on Al(III) in experimental animals was reported by Döllken in 1897. The scientific interest for the risk connected with the exposure of humans to aluminum uptake under most diverse conditions has growth exponentially in recent years*. Metal uptake from dinking water, food and beverages, pharmacological products, as well as as dust in working areas, antiacids, dialysis and enteral-parenteral fluids etc, has been evaluated and monitored. Apparently, living organisms are well and effectively protected from Al(III) aggression. It is generally accepted that only1% out of the daily intaken Al(III) enters the blood stream and about 90% of this amount is excrete by the renal function. Aluminum gains access to human body via two well established and one proposed pathways: the G.I. tract and the lung tissue, and the olfactory way. Abnormal exposure to A(III) is experienced by uremic patients and by occupationally exposed workers. The role of Al(III) as an etiopathological factor in the production of dialysis dementia and dialysis osteomalacia is now well established. In both cases, neurotoxic effect have been recognized, which can be effectively eliminated only upon prevention strategies. Critical inspection of the literature reveals that an abnormally high uptale of aluminum is associated with development of dialysis dementia, iron-adequate microcytic anemia, vitamin D-resistant bone disease and other. Al(III) has been implicated during the last two decades as a potential neurotoxic factor in different brain conditions, like for instance: 1. Dialysis encephalopathy 2. Alzheimer’s disease 3. Amiothrophic lateral sclerosis 4. Parkinsonism-dementia of Guam 5. Down syndrome with manifested Alzheimer’s disease features 6. Industrial exposure 7. Neurofibrillary degeneration adjacent to hemartoma 8. Striatonigral syndrome 9. Alcohol dementia with patchy demyelination 10. Senile plaques and neurofibrillary tangles in Azheimer’s disease 11. Aged human brain The phenomenological connection between aluminum focal accumulation in pathological features of Alzheimer’s disease (AD) like neurofibrillary tangles and senile plaques appears to be established by numerous scientific reports, and the amount of positive observations, in this connection, represents a strong impulse to the deepening of the knowledge on the molecular bases of aluminum neurotoxicity. The relevance of a dismetabolism of aluminum to the etiology and pathogenesis of AD is still a matter of intense debate and investigations. Biological, biochemical and bioinorganic models of aluminium toxicity are certainly convenient tools for sheding light on this most intriguing topic. AD is an exceedingly complex disease with manyfold histopathological and biochemical features and its is most likely a multietiological syndrome As a matter of fact, a huge amount of experimental work has been carried out in this and other laboratories on very many aspects of aluminium biology in the past two decades. However, it has become increasingly apparent that even the simplest experimentation with aqueous Al(III) has to face with the intrinsic complexity of the Al(III) speciation in neutral solutions and this subject has been recently reviewed by our research group in some comprehensive reviews. In pure phenomenological terms, the interaction between Al(III) with biological systems is documented by an impressive number of papers (ca. 1000/year). In spite of this abundant literature, direct information on the molecular bases of Al(III) biological activity are rather scanty. The reason for this unusual situation is at least due two main reasons:1) in the very large majority of the experimental toxicology protocols Al(III) was administered 7 either under ill-defined pH conditions or as ill-defined mixture of various chemical species in the neutrality range, and 2) very few research groups investigated on the specific biological acitivity of Al(III) administered under precise chemical forms and on with biologically relevant macromolecules. As a matter of fact, researchers are now challenged to interprete an enormous amount of biological, Al(III)-related, phenomena on the basis of a dearth of bioinorganic molecular information. A conceptual experimental breakthrough aimed to circumvent point 1) and 2) was independently proposed in our and other laboratories in 1986. The basic intuition is the employing of Al(III) complexes bearing: a. a defined molecular structure in solutions under the actual employment conditions b. net zero electrical charge c. high hydrolytic stability d.defined hydrophilic/hydrophobic character measured in n-octanol/water partition coefficients The combination of these requisites offers the possibility of using artificial toxicants for which it is possible to calculate the metal speciation when biological system is exposed to the synthetic toxicant. Thus, under these conditions, dose-response relationships become reliable and molecular models of Al(III) toxicity may be reliably developed. In the absence of a strong coordination agent, the aqueous solution chemistry of Al(III) is seemingly simples. The thermodynamically predicted species which dominate at pH 7.5 are Al(OH)4- (10-6.86 M) and Al(OH)3 ( 108.10 M) at an expected total metal concentration equal to 10 -7 M. Diversely complexes such as Al(acac)3 (acac= acetylacetonate) and Al(malt)3 (malt = maltolate) appear to meet the requisites necessary to by-pass the drawbacks outlined in points 1. and 2. The equation dealing with the metal speciation in water of artificial toxicants bearing bidentate monoionic ligands, are the following: Ki Al(L-L)3 + 6H2O <=> [Al(H2O)6]3+ + 3 L-L(1) 3 L-L- + 3H2O Kb <=> 3H(L-L) + 3OH- (2) Kb Al(H2O)63+ + 3OH- <=> Al(OH)3 + 6H2O (3) Kdec Al(L-L)3 + 3H2O <=> Al(OH)3 + 3Al(L-L) (4) As a paradigmatic example in order to confirm our model we investigated the effect of Al(III) speciation of blood-brain barrier (BBB) permeability by administering Al(acac) 3 (hydrolytically stable and lipophilic) and Al(malt)3 (hydrolytically stable and hydrophilic) by i.p. injection in rats. As expected, while the action of Al(III) on BBB was confirmed, new aspects of this biological effect became apparent. In fact, while Al(malt) 3 produced a transient effect lasting few hours, Al(acac)3 caused an almost irreversible increase of BBB permeability. Aluminum accumulation could be observed only in the neurons of the cerebral cortex from rats treated with the more lipophilic compound. Diversely, Al(malt)3 revealed positivity for aluminum only on the microvessels. Several other biological models were utilized to better understand the Al(III) speciation effect and its relevance in various physiological as well as biochemical pathways. The following table briefly summarizes the main findings on the effects produced by Al(III) in several biological targets as observed in our laboratory. SOME EFFECTS PRODUCED BY ALUMINUM(III) ON VARIOUS BIOLOGICAL TARGETS 1. 2. 3. 4. 5. 6. Inhibition of proteases (trypsin and -chymotrypsin) Activation of Acetylcholinesterase Activation of Hexokinase Activation of Na+/K/ATPase Inhibition of Ca2+/Mg2+ ATPase Modification of the activity of some enzymes of the Krebs cycle 8 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17 Modification of the activity of some lysosomal enzymes Alteration of the permeability of the blood-brain barrier Alteration of the cell membrane fluidity Alteration of intracellular calcium Alteration of mitochondria metabolism Alteration of lysosomal proton pump Alteration of the functionality of VDAC channels Neurotogenic effect on neuroblastoma cells Neurotrophic effect on nervous system at low concentrations Indirect stimulation cell membrane peroxidation Alteration of the activity of SOD, Glutathione peroxidase and Catalase Relevant books and Reviews from our laboratory Aluminum in Chemistry, Biology and Medicine (1991) Nicolini M., Zatta P., Corain B. (Eds). Raven Press, pp. 117. Aluminum in Chemistry, Biology and Medicine (1994) Nicolini M., Zatta P., Corain B. (Guest eds). Life Chem Reports 11: 1-267. Non-Neuronal Cells in Alzheimer’s Disease (1995) Zatta P. and Nicolini M.(Eds), World Sci., Singapore, pp.232. Aluminum in Infants’ Health and Disease. (1997) Zatta P. and Alfrey A., World Sci. Publ., Singapore, pp. 245. Alzheimer’s Disease and Related Disorders (1992) Nicolini M., Zatta P., Corain B. (Eds), Pergamon Press pp. 474. Aluminum Chemistry (1996) (Corain B., Bombi G.G., Nicolini M., Zatta P. (Guest eds) (Special issue) Coord. Chem. Rev. pp. 404. Aluminum Chemistry (2002) (Zatta P., Guest ed.) (Special issue) Coord. Chem. Rev. (In press). Further information can be seen on the Web site: www.bio.unipd.it/~zatta/aluminum.html * The number of scientific publications reported in Medline on aluminium per years is about 1200. ** Aluminium and Alzheimer’s disease. The science that describes the link. (2001) ((Exley, editor) Elsevier, Amsterdam, Holland. 9 b. METALLOTHIONEINS, AGING AND NEURODEGENERATIVE DISEASES In the human brain, aging is considered as one of the most relevant risk factors for neurodegenerative disorders and a state in which pathological alterations may exist without an apparent clinical expression. In addition, in aging brain, genetically programmed time-associated modifications in cells may increase their susceptibility to dangerous environmental factors like for instance, hormonal changes, infective diseases, immunological disorders etc., and consequently lead to a variety of pathological events observed in the aged brain. Free radicals are highly reactive chemical species with an unpaired electron in an atomic or molecular orbital. In biological systems in the presence of transition metals like iron, copper, manganese and others, the most relevant form of radicals are superoxide and hydrogen peroxide, that are dangerous in that they attack proteins, nucleic acids and membranes containing polyunsaturated fatty acids. Particularly in the brain free radicals can be produced by catecholamine mismetabolism especially when a deficiency of antioxidants occurs. In Alzheimer’s disease (AD) brain lesions are present that are typically associated with attacks by free radicals (e.g., DNA damage, protein oxidation, lipid peroxidation, advanced glycosylation end products, etc.) in the presence of metals that catalyze the production of such radicals. Neuroinflammatory phenomena are also important factors in neurodegenerative diseases in that they increase formation of reactive oxygen species (ROS) and nitrogen species that could be a major risk in the neurodegenerative development. Glial cells and astrocytes in particular, play a central role in the inflammatory phenomena, and astrocyte activation is a relevant marker for neurodegenerative disease like Alzheimer’s, Binswanger’s disease, Down’s syndrome etc. The brain in fact is considered very sensitive to oxidative damage in that is enriched in the more easely peroxidizable fatty acids and with about 20 % of the total O2 consumption with its 2% with respect to all whole body mass. In recent years considerable data support the hypothesis that the brain from AD subjects is under increased oxidative stress and this is considered as a relevant issue in the pathogenesis of neuronal degeneration. Amyloid -peptide, the major constituent of the senile plaques (SP) in AD brain, has been shown to be a source of free radicals. In the brain, metallotioneins (MT-I-II) were identified in pia mater, ependymal cells, astrocytes, glial processes and microcapillaries. In the cerebellum MT-I-II were identified in the Bergmann cells, and in the protoplasmatic astrocytes of the white matter. Among numerous hypotheses advanced to explain some neurodegenerative diseases the relevance of free radicals has been recently and strongly raised by some authors. In this connection the role played by astrocytes appear to be of paramount importance in that they generate the highest concentration of -amyloid of all cell type so far tested. In addition, reactive astrocytes increase the production of the amyloid precursor protein (APP) which in turn may recruit other reactive astrocytes and microglial cells to increase the APP production. It has been recently reported that in AD subjects cerebral white matter contains numerous MT-I-II-expressing astrocytes with an intense immunoreactivity of the cell body. MT participate in intracellular defense against ROS and nitrogen species. Chronic inflammation in AD has been postulated to raising the possibility that the etiology of AD have an immunological component. Cytokines and IL-1, for instance, elevated in AD, induce MT-I-II production in astrocytes suggesting that these proteins may have a relevant role in providing long-term protection against oxidative damage, injury and inflammation with a multiple compensatory mechanism involving the osmotic regulation of some metal ions. Thus, while it seems rather clear the overexpression of MT-I-II in the astrocytes from AD as well as from other neurological disorders, more controversial appears the presence, the role and the meaning of MT-III in connection with some neurological disorders. In AD, for instance, some authors observed that MT-III is down-regulated, while other claimed that the reduction in the expression of MT-III is most likely due to several other factors not necessarely linked to AD pathology. MT-III is growth inhibitory factor (GIF) particularly abundant in glutamatergic neurons that releases Zn from synaptic terminals, and in Zn-containing neurons of the hippocampus where it could play a role in neuromodulation in a way yet to be so far well understood. MT-III, besides in neurons, is also induced in astrocytes in the cerebral cortex in cases of meningitis, Creutzfeldt Jacob disease as well as in reactive astrocytes sorrounding cerebral infarct. It has been claimed also that GIF is reduced in a subset of reactive astrocyrtes in lesioned areas of degenerative diseases such as AD, multiple-system atrophy, Parkinson’s disease, progressive supranuclear palsy and amyothrophic lateral sclerosis in relation to neuronal loss. Noteworthy, MT-III deficient mice show decreased concentration of Zn in several brain regions and they are more susceptible to seizures induced sperimentally with consequent greater neurons injury. Conversely, transgenic mice with high level of MT-III are more resistant to hippocampal neurons injury induced by seizures. According to Masters et al. (1994), MT-III may participate in the utilization of Zn as a neuromodulator. In the central nervous system, Zn is present in all regions in a rather high concentration with a definit, however, not well defined role in neurochemistry. Zn interacts with enzymes and proteins including transcription factors which are critical for cell survival and could be linked to apoptotic processes. The concentration of Zn, Cu and other metal ions has been reported to be altered in several neurological disorders like Alzheimer’s, Parkinson’s, Huntington, Pick disease, alcoholism, schizophrenia, Down’s and Wernike-Korsakoff syndrome, amyotrophic lateral sclerosis and others. In addition, concentration of Cu, Fe and Zn were measured in the rims and cores of SP and in the neuropil of the amygdala of AD patients. Both an excess or a deficiency of Zn have been reported to be associated with neurological diseases. Zn loss is associated with anorexia, hyposmia, cerebral dysfunction and it has potent inhibitory action on 10 glutamate decarboxylase. It serves in a double role in initiating amyloid deposition in AD and then being involved in mechanisms attempting to quench oxidative stress and neurotoxicity derived from amyloid mass. Zn has a role as a neurosecretory product highly concentrated in synaptic vesicles of a specific contingent of neurons that represent a subset of glutamatergic neurons. Related to AD, the APP is proteolitically processed by an secretase to release a soluble form of an APP derivative and interestingly to note -secretase is a Znmetalloprotease. In addition, soluble human amyloid binds specifically Zn and rapidly becomes a strongly insoluble compounds which easely aggregates. In this connection, although, a single essential function of MT has not been found yet, MT evolved as a mechanism able to regulate Zn levels and its distribution inside the cell. The ubiquitously essential distribution of Zn coincides with the ubiquitous distribution of MT-I-II. These proteins are produced by reversible activation of Zn interaction with Zn-finger domain of the metal-responsive transcription factor (MTF) which activates MT-gene in response to metals and oxidative stress. MTF is thus a sort of sensor of free Zn pool in the cell which can change in response to chemical diverse inducers. Regulation of MT genes by metals and oxidative stress involves multiple signaling pathways which depend on the species of metal ion and the nature of the oxidative stress. Thus, Zn metabolism could be relevant in the brain in the neuropathogenesis of AD and other neurological disorders through the physiopathological expression of MT. In conclusion, the role of MT, generally speaking, is yet to be fully understoood, however, it appears that while MT-I-II are induced in relation to the progression of the age-related modifications in the brain and they play an important role in the protection of tissue from toxic insults (e.g., free radicals, metal ion mismetabolism) responsible for the brain aging; diversely, MT-III could play a role in maintainance of Zn-related essential brain functions. References Hidalgo J., Aschner M., Zatta P., Vasak M. (2001) Roles of the metallothionein family of proteins in the central nervous system. Brain Res. Bull. 55: 133-146. Mocchegia E., Giacconi R., Cipriano C., Muzzioli M., Fattoretti P., Bretoni-Freddari C., Isani G., Zambenedetti P., Zatta P. (2001) Zinc-bound metallothioneins as potential biological markers of ageing. Brain. Res. Bull. 55: 147-155. 11 c. ALUMINUM AND THE BIOPHYSICAL STATE OF CELL MEMBRANE In the last ten years a lot of progress has been made in a better understanding the properties of aluminum with respect to its impact in the biosphere. However, in spite of an enormous number of scientific papers on the chemistry, bioinorganic, biochemistry as well as toxicology of aluminum, it is still premature to define the precise molecular mechanism/s that could fully explain the influence of this metal ion on the biological targets, including cell membranes. Experimental data confirm that aluminum is able to deeply alter the molecular structure of the lipid bilayer, modifying thus, biophysical and physiological properties of the cell membrane such as fluidity, rigidity, transduction of signals, channel permeability and protein activity. Furthermore, to complicate more the scenario, a great deal of differences has been found between data obtained from in vitro and in vivo experimentation. Often, results obtained by in vivo models are more dramatically relevant than those obtained using in vitro models, suggesting a more holistic action of aluminum toward biological targets. A better understanding of the role of the metal speciation appears to be one of the most important issues to be studied in order to understand aluminum toxicological properties. This aspect is extremely important if it is considered that aluminum is present everywhere in our everyday life, like in food, beverage and pharmaceutical products. In relation to a possible connection between aluminum and Alzheimer’s disease, while a directed connection with the etiopathogenesis of this disease is still to be proven, several elements seem to indicate that the metal ion could be somehow involved concurring in the aggravation of some pathological events observed in this devasting pathology, especially in those individuals more prone to aluminum uptake, on the bases of the following observations: -Aluminum produces a profound alteration on the biophysical status of biomembranes, consequently, it can induce a strong modification of cell membrane anysotropy alterating strategic physiological functions on the lipid metabolism as well as on the biochemical and physiological properties of proteins associated to the lipid bilayer. -The higher presence of phosphatidylcholine derivatives in the cell membranes of AD (Pettegrew et al., 1984; Wurtman et al., 1990), could facilitate an Al3+ overaccumulation in the surface of membranes. In addition, the hyperphosphorylated tau proteins associated to the cytoskeleton, as observed by several authors in AD, is an elective place for Al3+ accumulation as hypothesized by Zatta (1995) and partially proven (Shin et al., 1995; Shin et al., 1997) with a consequent possible blockage of the axonal flux (Zatta, 1995). -The interaction between Al and biomembrane produces a significant rigidification that could contribute to a reduction of the width of the lipid bilayer as it has been observed in AD by Mason et al.,(1993), favoring thus the abnormal exposure of the amyloid segment of the APP to a more strategic proteolysis by some putative yet to be discovered secretase. -Al altering membrane biophysical properties of mitochondria and ER, and inhibiting Ca 2+-ATPase activity, (Mirzabekov et al., 1993; Gandolfi et al., 1998) can potentially alter Ca 2+ homeostasis influencing apoptotic phenomena. -The observed structural destabilization of the cell membrane by Al could induce a more relevant production of free radicals by the transition metals, mainly Fe(II) increasing oxidative phenomena (Zatta et al., 1989; Zatta et al.,1997; van Rensburg et al., 1992) as observed in AD. -Al acting as a membrane destabilizer at the BBB level can deeply modify its permeability as it has been observed in experimental animals in a speciation-dependent way (Favarato et al., 1992). Further studies are thus necessary to better understand the role of Al in connection with AD, if any. It is a fact that the molecular, biophysical and chemical bases of individual sensitivity to aluminum, and the consequent involvement as a potential risk factor or at least as an aggrvating cofactor for AD, as well as for other neurodegenerative diseases, is still an issue to be explored in the near future. REFERENCES Favarato, M., Zatta, P., Perazzolo, M., Fontana, L., Nicolini M., (1992) Aluminum (III) influences the permeability of the blood-brain barrier to [14C]sucrose in rats. Brain Research 569, 330-335. Gandolfi, L., Stella, M.P., Zambenedetti, P., Zatta, P. (1998) Aluminum alters intracellular calcium homeostasis in vitro. Biochimica & Biophysica Acta. 1406, 315-320. Mason, R.P, Shoemaker, W.J, Shajenko, L., Herbette, L.G (1993) X-ray diffraction analysis of brain lipid membrane structure in Alzheimer’s disease and beta-amyloid peptide interactions. Annals of the New York Academy of Sciences 24, 54-58. 12 Mason, R.P., Trumbore, M.W., Pettegrew, J.W. (1996) Molecular membrane interactions of a phospholipid metabolite. Implications for Alzheimer’s disease pathophysiology. Annals of the New York Academy of Sciences 777, 368-373. Mirzabekov, T., Ballarin, C., Nicolini, M., Zatta, P., Sorgato, M.C. (1993) Reconstitution of the native mitochondrial outer membrane in planar bilayer. Comparison with the outer membrane in a patch pipette and effect of aluminum compounds. Journal of Membrane Biology 133, 129-143. Pettegrew, J.W., Kopp, S.J., Dadok, J., Minshew, N.J., Feliksik, J.M., Glonek, T., Cohen, M.M. (1984) Chemical characterization of a prominent phosphorylmonoester resonance in animal. Huntington’s and Alzheimer’s brain: 31 P and 1H NMR analysis at 4.7 and 14.1 tesla. Annals of Neurology 16, 136-138. Shin, R.W., Lee, V.M., Trojanowski, J.Q. (1995) Neurofibrillary pathology and aluminum in Alzheimer’s disease. Histology and Histopathology 10, 969-978. Shin, R.W., Lee, V.M., Trojanowski, J.Q. (1997) Aluminum modifies the properties of Alzheimer’s disease PHF tau proteins in vivo and in vitro. Journal of Neurosciences 14, 7221-7233. Van-Rensburg, S.J., Carstens, M.E., Potocnik, F.C., Aucamp, A.K., Taljaard, J.J., Koch, K.R. (1992) Membrane fluidity of platelets and erythrocytes in patients with Alzheimer’s disease and the effect of small amounts of aluminum on platelets and erythrocyte membranes. Neurochemical Research 17, 825-829. Wurtman, R.J., Ulus, I.H., Blusztajn, I., Lopez G., Coviella, I.L., Longue, M., Mauron, C., Growdon, J.H. (1990) Choline levels the regulation of acetylcholine and phosphatidylcholine synthesis, and Alzheimer’s disease. In Alzheimer’s disease. Epidemiology, Neuropathology, Neurochemistry, and clinics. (Eds. K. Maurer, P. Rieder, P. Beckmann) Springer-Verlag, Wien, New York, 211-224. Zatta, P., Perazzolo, M., Bombi, G.G., Corain, B., Nicolini M. (1989) The role of speciation in the effects of aluminum (III) on the stability of cell membranes and on the activity of selected enzymes. In Alzheimer’s disease and related disorders. (Eds. K. Iqkal, H.M.Wisniewski, B. Winblad) Alan Liss, New York, 1087-194. Zatta, P. & Nicolini, M. (Eds) (1995) Non-Neuronal cells in Alzheimer’s disease. World Science. Singapore, London. Zatta, P., Zambenedetti, P., Toffoletti, A., Corvaja, C., Corain, B. (1997) Aluminum(III) induces alterations on the physical state of the erythrocytic membrane: an ESR evaluation. Journal of Inorganic Biochemistry 65, 109114. Zatta, P., Nicolini, M. (Eds) (1995) Non-Neuronal cells in Alzheimer’s disease. World Science. Singapore, London. 13 2. OXIDATIVE STRESS INDUCED BY PHOTODYNAMIC EFFECTS ON THE STRUCTURE AND FUNCTION OF CELLS AND SUBCELLULAR ORGANELLES This research project was aimed at the understanding of the mechanisms of photosensitization, i.e. the process induced by highly toxic, reactive oxygen species (ROS), produced after irradiation by UV-visible light of some fluorescent molecules (in particular, porphyrins and analogous compounds, such as phthalocyanines, naphthalocyanines etc.) that is involved in some types of cell damage and death. Porphyrins have been widely studied as sensitizers of living organisms in the light-promoted reactions. The porphyrin-mediated photodamage is mainly due to the production of singlet oxygen ( 1O2), through an electronic energy transfer from the first excited triplet state of the sensitizer to the molecular oxygen in its ground state (triplet). The photosensitization process can be highly noxious and cause irreversible cell damages, as observed in human porphyria or in skin lesions such as erythema and cancer. However, porphyrins and other photosensitizers have found useful biomedical applications in the treatment of several deseases, as, for ex., in the photodynamic therapy of tumors (PDT) and in the destruction of atheromatous plaques and psoriatic lesions. Pioneering studies concern the use of photosensisting molecules as antibacterial and antiviral agents. Most porphyrins which have been tested both in vitro and in vivo carry on their photoactivity after preferential association to membranes (in particular, plasma, mitochondria and endoplasmic reticulum membranes), and the targets of photodamages are proteins and lipids. The extent of photodamage mainly depends on the sensitizer aggregation state (only monomeric species are photoactive) and its distribution pattern into cells and subcellular organelles. The maximum photoprocess efficiency, in fact, is reached when photosensitizer and target are localized in close proximity, being the diffusion in biological material) owing to their high reactivity. This research was focused on the characterization of the photochemical and photophysical properties of photosensitizing agents in model and biological membranes as well as on the study of their uptake and photodamage mechanisms on functional activities of cells and subcellular organelles. Particularly studied was the correlation between the distribution properties of porphyrins and derivatives and their photosensitizing efficiency in mitochondria, which are primary targets of the photodynamic effect in vivo. The distribution pattern of these molecules in membranes is influenced by several factors including the degree of dye hydrophobicity, the nature of the carrier and the incubation time with the membrane. As an example, hematoporphyrin (HP), a photosensitizer characterized by a moderate degree of hydrophobicity, preferentially partitions to protein sites of the inner mitochondrial membrane (IMM), which exhibits a higher protein content (21% of total protein) as compared to the outer membrane (OMM , 5% of protein). It has been postulated that the HP-binding sites are localized on the ADP/ATP translocase: actually, at mild irradiation conditions, the most sensible function to the HP photodynamic action is the ADP transport whereas other enzymes involved in the ATP synthesis, such as the ATP synthase or the Pi carrier as well as the enzymes of the respiratory chain and the Ca 2+ transport are less affected. The enzymes of the outer membrane, matrix and intermembrane space require massive light doses for the photodegradation. HP distribution pattern in mitochondria is not affected by the nature of the transport system to the membrane. More hydrophobic dyes than HP, such as protoporphyrin (PP), localize in mitochondrial lipid domains and their distribution properties are largely modulated by the chemical composition of the carrier and the incubation time. At short incubation periods (<2 min ), liposome-bound PP accomodates in lipid domains of the IMM provided that the liposomal carrier is enriched in cardiolipin; in lipid domains of the OMM if the dye is transported by cholesterol-rich liposomes. Thus, PP-uptake by mitochondria is controlled by the affinity in lipid composition between the carrier and the acceptor membrane. The dependence of the uptake properties on the lipid composition of the carrier is lost after incubation periods longer than 2 min, due to a migration of PP to IMM. In the latter case, the extent of ADP transport inhibition by irradiated PP approaches that shown by HP. Highly hydrophobic dyes, such as Zn2+ -phthalocyanine (ZnPc), interact predominantly with lipid domains of the OMM indipendently of the transport system and incubation time. The transport system of photosensitizers in vivo is a crucial determinant. A pimary goal in the photodynamic treatment of tumours, for ex., concerns the achievement of tumor selective targetting by the photosensitizer. To this aim, most researches are oriented to obtain the specific release of the drug to the low density lipoproteins (LDL). It has been credibly hypothesized that, in vivo, LDL play the role of preferential carriers of the sensitizer to tumoral tissues. In fact, LDL interact with several types of tissues, in particular hyperproliferating tissues, via an endocytosis-mediated process. In agreement, LDL-associated photosensitizers administered to tumor-bearing rats accumulate preferentially in malignant tissues as compared to the drugs freely dissolved in aqueous solutions or incorporated in other lipoproteins lacking of specific receptors on plasma membranes. The selective sensitizerserum lipoproteins interaction is obtained after incorporation of the drug in liposomes. In fact, while aqueous HP or HP dimethyl esther or ZnPc for ex., distribute into at least three classes of serum proteins (albumins, globulins, 14 lipoproteins), a selective association to lipoproteins is obtained after incorporation of the drug in liposomes of dipalmitoyl phosphatidylcholine (DPPC). The efficiency of the drug release process depends on the chemical composition of the carrier. Liposomes carrying phospholipids characterized by shorter hydocarbon chains, such as dimiristoyl phosphatidylcholine (DMPC) , lead to an aspecific distribution of the sensitizer in different serum proteins. Recently, this research has been focused on the effects of the photosensitization-mediated oxidative stress on the permeability transition (PT) process of the mitochondrial inner membrane, that has been postulated to be involved as early step in the cascade of events leading to apoptosis or programmed cell death. The transition from the impermeable to the permeabilized state of the inner membrane after Ca 2+ accumulation by mitochondrial matrix is characterized by the opening of a cyclosporin A-sensitive proteinaceous pore that leads to mitochondrial uncoupling, matrix swelling, permeation to solutes with molecular masses up to 1500 D, Ca 2+ efflux and loss of matrix components. This program can be subdivided into the following three lines : a. Relationship between the photosensitizer microenvironment and its photosensitizing properties.. One of the major parameters that influence the sensitizer photoactivity in biological membranes is the nature of its microenvironment after incorporation in the membrane. Inserting the sensitizer deeper within the hydrophobic membrane bilayer increases the dwell time of singlet oxygen in the membrane ( 1O2 in apolar media have been reported) whereas a partition to the lipid/water interface or to the aqueous phase leads to a very rapid decay of 1O2 which is quickly deactivated by solvent molecules (1O2 lifetime in H2O is about 3 s). Therefore, in cellular systems the 1O2 -induced photodamage is higher when both sensitizers and targets are localized in the membrane hydrophobic regions rather than in the cytoplasm or in the external medium. The maximum effect will be obtained when sensitizer and target are situated in close proximity. The molecular nature of the photosensitizer microenvironment determines the type of the final target of the photosensitization process thus leading to different cell responses to the photodamage. On these basis, it is very important to search for those conditions that could permit to address the sensitizer to a particular microenvironment thus discriminating the photodamages on different cell functional activities. As an example of this approach, it has been shown that irradiation of mitochondria in the presence of HP produces 1O2-mediated oxidative effects that lead to inhibition of the mitochondrial PT. This effect is quite unexpected since it seems a general rule that oxidative stress, independently of the reaction mechanism of the different oxidative species, cause induction of the PT. The effect has been attributed to degradation of critical histidines, present in one of the regulatory sites of the PT process. In contrast with what observed for HP, trimethylpsoralen (TMP), a structurally different molecule whose photoactivating effect is also mediated almost exclusively by 1O2 , induces opening of the PT pore after irradiation as a consequence of critical thiols oxidation and formation of cystine, a reaction that increases the probability of the PT process. Although both HP and TMP are bound to proteins of the inner membrane. it has been ascertained that their interaction sites are different. This research has demonstrated that the same reactive species can activate or inhibit a cell function (as the mitochondrial PT) depending on the site where it is produced in a biological membrane. b) Generation of reactive oxygen species by photoactivated cell-probes and implications for studies of cellular functions. Because of the key role performed by mitochondria in cell metabolism as well as in the regulation of normal cell functions of particular interest is mitochondrial photosensitization. On these bases, photosensitisers specifically targeted to mitochondria may provide versatile applications in studies of mitochondrial contribution to processes leading to cell death. On the other hand, photochemical effects are undesirable when fluorescent probes are used for studies of cell viability and microscopic imaging. Microscopic observations using epifluorescence or laser illumination in confocal microscopy may result in the perturbation of cellular structures and induction of severe cell damage if dye concentrations and exposure times to the light are not properly tested. Some of these probes have been demonstrated to be highly photoreactive and lead to cell apoptosis after exposure to light sources used in microscopy studies. By our studies, calcein, a water-soluble, polyanionic fluorescein derivative which is currently used for probing membrane integrity and permeability and in studies of mitochondrial permeability transition in cells by microscopy fluorescence imaging, was recently found to induce photosensitized alterations in isolated mitochondria thus suggesting potential artifacts in confocal microscopy studies of cells. These results suggest that a thorough investigation should be performed on the possible photosensitizing effects elicited by other fluorescent probes. In particular, various potentially phototoxic cationic dyes, such as rhodamines, are used to measure the mitochondrial membrane potential (m) at the single cell level, in conjunction with microscopy techniques . The prolonged exposure time required to obtain good 15 resolution can dramatically affect both cell viability and mitochondrial function. This problem is worsened in the case of confocal microscopy, where cells are exposed to the high intensity of laser beams. Because of their phototoxic effects, potentiometric fluorescent probes are in fact under investigation as potential therapeutic agents against various types of tumors , which appear to accumulate these molecules more efficiently than normal, non transformed cells. Thus, the fluorescent probes could contribute to cause or potentiate rather than simply measure the changes of m. On these basis, work is in progress to define the experimental conditions which allow a safe use of these probes in intact cells. c) HP-fluorescence anisotropy studies in relation to the mitochondrial permeability transition. Induction of the mitochondrial permeability transition (PT) gives also rise to modifications in the membrane dynamic properties. In the lipid regions of the bilayer such changes arise only from the modulating effects of the PT-induced osmotic swelling and/or membrane potential decay, as sensed by the fluorescence anisotropy changes of 1,6-diphenyl-1,3,5-hexatriene (DPH) for highly hydrophobic domains and 1-anilino-8-naphthalene sulfonate, (ANS) or 1-[4-(trimethylamino)phenyl]-6-phenyl-1,3,5-hexatriene (TMA-DPH) for the phospholipid headgroup areas. Studies of the PT-induced anisotropy changes of hematoporphyrin (HP) as reporter of membrane protein regions indicate that the above-mentioned effectors cannot account alone for the total PT-effect and the protein alterations can be mostly attributed to conformational variations of the HP-binding sites during the assembly of the PT pore. The specificity of HP in sensing changes in membrane structure during pore opening was ascribed to a strategic location of the dye in protein sites of the inner mitochondrial membrane which appear to participate in pore formation or regulation. This interpretation is strenghtened by comparative studies of mitochondrial interaction with HP and a structurally different fluorescent probe, namely 3,4´,5-trymethylpsoralen (TMP). Both dyes bind to inner membrane protein sites; however, the TMP-anisotropy variations during opening of the PT pore reflect only structural changes induced by the potential decay. The strict relationship between the dynamic properties of HP-binding area and the conformation of the pore complex is further supported by the fact that similar changes of HP-anisotropy are observed after triggering the pore on different key sites which are activated by various inducers (FCCP, PhAsO, DIA etc.). On these bases, HP fluorescence anisotropy can be considered a promising tool to monitor the PT pore characteristics, because it directly follows the conformational properties of the pore constituents rather than the effects of the pore produced on the whole mitochondrial structure (swelling, potential decay, loss of calcium, release of matrix components). 16 3. SOVRAMOLECULAR ORGANIZATION, FUNCTIONAL REGULATION AND BIOINORGANIC PROPERTIES OF INVERTEBRATE OXYGEN CARRIERS Why invertebrate oxygen carriers? Invertebrate oxygen carriers are unique macromolecules. Their peculiarity mainly resides in the oligomeric structures that exhibit high levels of complexity. Proteins from organisms that belong to different phyla have quaternary structures that can be related to distinct topological models. Within the same phylum, however, the different structures are related to the same topological model. Different kinds of subunits constitute the different functional aggregates. This large structural differentiation manifests itself in a significant functional flexibility whose description requires allosteric models of increasing complexity. The ultimate aim of this research program is to study the correlation between the structural modification at the active site induced by oxygen binding and the changes in shape of subunits and of the whole molecules; on these changes is based the cooperativity of these proteins. To reach this aim we have integrated different experimental approaches that allow for the investigation on different dimensional scales. In invertebrates four classes of oxygen carriers can be singled out on the basis of their prosthetic groups: Hemoglobins (Hb, heme-proteins), Chlocruorins (Chl, chlorocruoro-heme proteins), Hemerithrins (Hr, non-heme iron) and Hemocyanins (Hcs, dinuclear copper). The molecular complexity is different within the four classes, yet a precise description of the functional regulation mechanism is still missing even in the case of the simplest cases. We want to establish the relationships between their functional properties and the structural modifications in the active site region induced by oxygen binding to the various aggregation states that are characterised by different affinities for this ligand. Furthermore, we also address the role of the polypeptide chains heterogeneity on the allosteric properties. Our approach to study such structure-functions relationships involved different levels of complexity. One level of complexity concerns the presence in such proteins of metal ion(s) that are of fundamental importance for coordinating molecular oxygen. As outlined above, the evolutionary strategies have selected copper and iron in different complexes (iron-protoporphiryn or dinuclear sites). In Hbs, the reactivity of the metal prosthetic group is strongly dominated by the presence of the porphyrin ring that involves four out of six coordination positions of the metal ions. A great deal of work is available in literature on the dependence of the affinity for dioxygen as a function of: - distortions of the tetrapyrrol ring, - bonding distance between iron and proximal histidine, position of the iron with respect to the plane defined by the tetrapyrrol nitrogens, - position of the distal histidine with respect to the iron. Hc and Hr have dinuclear metal active sites where the two metal ions, directly bound by the protein matrix, are not equivalent in their reactivity and accessibility to exogenous molecules such as dioxygen, although in both cases the reaction between the O 2 molecule and the bimetallic centre is a reversible redox process. The second level of complexity involves the whole molecule where the oligomeric composition and the hierarchical interactions among different subunits play fundamental roles for the onset of cooperative phenomena and for the functional modulation as a response of external stimuli. Hcs have an active binuclear site with copper of type 3, directly bound to six histidine nitrogens of the protein chain and located within hydrophobic pockets, shielded from the external media. Crystal structures are available for a 50 kDa functional unit Odg from Octopus dophleinii for subunits a and b of Panulirus interruptus and for subunit II of Limulus polyphemus. In arthropod Hcs the two copper(II) ions, labelled CuA and CuB, are bound by three histidine residues each that are provided by a total of four different α-helical segments with antiparallel orientation with respect to each other. An additional difference is observed in mollusc Hc where the Cu A is coordinated to one histidine of a α-helix and to two histidines of a loop region, one of which is involved in an unusual thioether bridge. The molluscan CuB, similarly to the CuA and CuB in the arthropod Hcs, is coordinated by the histidines of a two α-helices motive. CuA and CuB are not equivalent and play different roles during the biological function. The CuA copper(II) centre, that is more accessible in the binuclear site, controls the reactivity of the site, while the second copper(II) centre (CuB) plays a complex role in controlling the local conformation and providing electrostatic effects during the oxygenation cycle. Deoxy-Hc contains a di-Cu(I) active site, whereas oxy-Hc is described as a -2:2 di-Cu(II)-peroxo complex. This peculiar bridging coordination mode is responsible for the onset of an intense absorption band around 340 nm (~20,000 M-1cm-1 ) attributed to peroxide-to-Cu(II) LMCT transitions. The unequivalence of the two metal ions is very evident in the case of the di-iron site of Hr. In this case, the protein provides three and two histidine nitrogens for binding Fe1 and Fe2 respectively. The two metal ions are bridged by two carboxylates and by a further ligand that, in the 17 deoxy-form, is an hydroxo-bridge for the two Fe(II). Dioxygen is bound as hydroperoxide to Fe2 and stabilized by hydrogen bonding to an oxo-bridge between the two Fe(III) ions. Such crystallographic evidences are in line with the solution studies on the non-equivalent reactivity of the copper ions in Hcs that represented one of the main research interests at the CNR Centre. Bioinorganic properties of the dinuclear copper site in hemocyanins We have studied the rather complex chemistry of the Hc active site with respect to a number of reactions that proved to be useful to probe the non-symmetric reactivity of the binuclear active site. Such reactions involve coordination of exogenous ligands that are reversible as in the case of non ionic ligands like CO, thiourea, thiocyanate, or promote specific reactions such as: copper removal, copper oxidation, electron transfer. In this frame it has been demonstrated that the two copper ions are not kinetically equivalent with respect to reactions that either change the oxidation state of the metal or remove the metal from the active site. Although the dinuclear complex itself possess several elements of symmetry the cavity provided by the protein matrix imposes a preferential path for the access of exogenous molecules. This non-symmetric exposure of the dinuclear metal complex strongly affects the overall reactivity of the active site which is dominated by the metal ion occupying the more exposed binding site (fast reacting site, FR-site), being the copper ion in the other site (slow reacting site, SR-site) substantially shielded. Therefore, several studies describe a variety of reactions that yield derivatives where the copper ion in the FR-site reacts specifically and only under more drastic conditions the reactions involve both metal ions. The non-symmetric exposure of the dinuclear copper complex to the solvent plays a role in the kinetics of copper removal by CN-, where the reaction has been described by a sequential mechanism with marked differences in the reactivity of the two copper ions, to give first, a partially metal depleted derivative, the ( --- , CuSR) semi-apo form and then the apo-form. The fluorescence properties of Hc are an important spectroscopic probe that can be used to discriminate between the binding of the copper ion to the SR-site and the FR-site. A significant quenching effect on the intrinsic fluorescence of Hc is observed only upon copper binding to the FR-site, while no influence can be attributed to the metal ion in the SR-site. This observation can be rationalised by the presence of one tryptophan residue close to only one of the copper in the crystal structure of Panulirus interruptus Hc. On the basis of spectroscopic evidences it is now possible to infer a correspondence between the crystallographic and the kinetic definition of the copper binding positions within the active site interpreting the fast reacting site as Cu A and the slow reacting site as CuB. It is worth noting that the non-equivalent exposure of the two metal ions in the active site derives from a structural characteristic of the protein matrix itself and, hence, the difference in the reactivity is maintained also when the binding sites are metal-depleted as in the apo-form. Consequentially, also the binding of metal ions to the apo-form may follow a kinetic mechanism that implies consecutive reactions, in which the first metal ion binds at the FR-site and then moves to the SR-site, living the FR-site again ready to bind the second metal ion. Thus, several procedures have been developed in order to obtain metal binding to the apo-Hc form. Cu(I) ions can reconstitute native Hc under a variety of experimental conditions. Also Co(II) ions, that have ionic radius similar to that of Cu(II), can be coordinated by apo-Hc yielding either mononuclear or binuclear metal substituted derivatives where Co(II) is specifically bound either to the FR-site or to both sites. Furthermore, the Cu(I) ion of the FR-site of deoxy-Hc can be directly exchanged with Co(II) yielding the specific mixed metal [Co(II)FR-Cu(I)SR] derivative. A mononuclear FR-site derivative has been prepared using Cd(II) ions which have a ionic radius quite similar with that of Cu(I). As observed for copper-containing Hc forms, the fluorescence emission of the metal substituted derivatives is affected by metal binding in the FR-site. The non-symmetric exposure of copper ions to exogenous molecules is evident also when the reactivity of copper ions is probed with respect to oxidation reactions under controlled conditions. A semi-met-Hc derivative can be prepared by selective oxidation one Cu(I) ion of the dinuclear cuprous site of deoxy-Hc with NO2 whit production of nitric oxide. A noteworthy aspect of this reaction scheme is its analogy to the single-electron reduction of nitrite catalysed by nitrite reductase where the type 2 copper site is involved in substrate binding. The catalytic competence of the dinuclear copper site of Hc in redox reactions has been demonstrated also in the case of hydrogen peroxide dismutation - reaction that can be conveniently used to prepare the dinuclear cupric derivative or met-Hc - and in the case of o-diphenol oxidation to quinines. The latter reaction, that is typical for other dinuclear copper containing proteins tyrosinases and polyphenoloxidases, has been recently interpreted as depending on low efficiency interactions between the o-diphenol substrate with the active site. The equilibrium position of the protein-substrate interaction can be modified, hence increasing the efficiency of the reaction, by introducing suitable conformation perturbants such as salts of the Hofmeister’s series, or by proteolytic cleavage of the native protein that result in an increased accessibility of the active site. The met-hemocyanin form, characterised by an EPR-silent [Cu(II) Cu(II)] site, is an important derivative for defining the structural characteristics of the active site in the native protein and to diversify the chemical reactivity of the two Cu-sites. The reaction of conversion of oxy-Hc to the met-derivative occurs spontaneously, but very slowly; however it can be stimulated by various anions including fluoride, azide and acetate. The 18 proposed active site structure for the met-Hc form assumes a Cu(II) binuclear derivative having an di--hydroxo bridge. A partial protonation of these bridges takes place at low pH. The fundamental question of the origin of the diamagnetism in the met-hemocyanin form is still unresolved. Azide is the best suitable exogenous ligand for probing the characteristics and accessibility of the active site in various met-Hc derivatives since, in coordinating with Cu(II), azide produces a complex with a moderate absorption in the 350-440 nm range (~1500-2000 M1cm-1) caused by the LMCT transition N - to-Cu(IIhe characteristics of this transition (position and 3 intensity) are strongly dependent on the mode of coordination of the ligand anion. The absorption and CD LMCT features of the azide-Cu(II) adduct allow to discriminate between terminal and bridging binding modes of the ligand. Concerning azide coordination, the Hc from the mollusc Octopus vulgaris and the arthropod Carcinus aestuarii met-Hcs differ from each other, pointing to specific differences that depend on the phylum. The affinity towards azide, the stoichiometry of binding, as well as its coordination mode show pH dependence. At pH 7.0, the same 1:1 stoichiometry of the azide adducts is exhibited by the two met-Hcs. However, the LMCT features in absorption and CD spectra suggest that azide binds in a bridging mode in the case of Octopus met-Hc active site in contrast to Carcinus met-Hc where azide binding, probably, occurs on the more exposed Cu(II) A centre in terminal mode. The suggested greater rigidity of the active site in the arthropodan case as compared with the molluscan met-Hc, may prevent the accommodation of azide as a bridging ligand inside the cavity containing the active site. The stoichiometry of binding of the azide ligand between the proteins of two phyla differs at pH 5.5. At this pH a second azide binds to Octopus met-Hc. The LMCT features in absorption and CD spectra are indicative of a bridging binding mode for the first azide (with greater affinity compared to pH 7.0) and a terminal binding mode for the second azide to copper (CuA) that controls the active site reactivity. The hemocyanin from arthropod at pH 5.5 behaves differently and binds only one azide molecule. Assuming for both met-Hcs the same bis-hydroxo adduct of the binuclear site, different reaction models for the binding of azide to Octopus and Carcinus met-Hcs have been proposed. High resolution Cu K-edge spectra provide a suitable tool to investigate the modifications of the coordination geometry of either Cu(I) or Cu(II) in the active site that are expected for the proteins exhibiting different reactivity. Two different approaches can be used in the analysis of the edge data: in a first one, is based on an established empirical correlation between the spectroscopic features of the XANES spectra and the coordination geometry of a large number of Cu(I) and Cu(II) complexes. A second approach for the analysis of the edge data implies their simulation using ab-initio calculation programs that uses, as input, structural models. The determination of the Cu-N bond length and the metal-metal distance are carried out by EXAFS spectroscopy. Comparison between data of the Hcs derivatives and of the related model compounds, provide results that support a description of the met derivatives as five-coordinated O-bridged dicopper(II) centre for the two proteins. The hypothesis of a bis(hydroxo) structure for both species, with a Cu-Cu distance about 3 Å is the favourite. The results obtained for the met-azido Hcs are compatible with the spectroscopic characterizations. The -1,3 mode appears more probable supporting the results based on optical spectroscopy. The situation of Carcinus derivative is more complex and less clear, however, a terminal mode seems more favourable. The X-ray absorption spectroscopy techniques has been applied also for the characterization of the dinuclear Cu(II)-peroxide site of oxy-Hc as well as of the dinuclear Cu(I) site of deoxy-Hc in a wide study aimed to correlate the coordination geometry at the active site level with the oxygen binding properties of the protein. This approach is particularly suited to study the Cu(I) complex of deoxy-Hc since it is devoid of optical properties and the reported luminescence assigned to the Cu(I)-imidazole complex is not as well characterized as to allow for structural resolution. Current knowledge on the biochemistry and the molecular physiology on Hcs indicate as plausible structural modifications deriving from oxygen binding to the dinuclear centre of the individual subunits or functional units: a) changes in bond length and coordination geometry for one or both the metal ions due to the decrease in ionic radius and to the increase of charge on going from Cu(I) (r=0.96 Å) to Cu(II) (r=0.72 Å); b) changes in the metal-metal distance; c) local conformational rearrangements of the active site region that change the accessibility of the metal for oxygen due to a) and/or b). On these bases, we believe that it is of great interest to verify if the same molecular mechanism can be used to describe the allosteric phenomenon observed in various Hcs, deriving from different species, which show only a limited sequence homology. The measurements require a controlled medium in order to avoid photoreduction of copper as it has been successfully accomplished by embedding the protein in a sucrose matrix. Such method has been proposed of general use for XAS spectroscopy on copper or iron proteins. Several arthropodan and molluscan hemocyanins have been studied in order to trace the inter- and intra-phyla differences. The XANES spectra of oxyhemocyanins of the different species are remarkably similar, consistent with a very strongly conserved coordination geometry of the copper active site. In contrast, small but significant differences are observed between the deoxy-forms of arthropodan and molluscan proteins. In particular, the XANES spectra of deoxy-arthropodan hemocyanins (with the exception of L. polyphemus Hc) show a more intense edge feature at approximately 8983 eV. This difference is tentatively assigned to a more planar geometry of the copper-ligands system in the 19 arthropodan rather than in the molluscan proteins. The first shell analysis of the EXAFS modulation is consistent with the presence of n = 3 N2 imidazole nitrogens at an average distance of 1.92±0.03 Å from copper in all the deoxy-hemocyanins investigated. Binding of dioxygen results for all hemocyanins in the increase of the number of first shell back-scattering atoms to n = 5 with average distances of 1.93 Å. Alternatively, by separating the contribution of N2 imidazole nitrogens and of peroxide O-atoms, n = 3 ligands at 1.98±0.03 Å and n = 2 ligands at 1.87±0.03 Å are found. These studies allow to propose that the different affinities for dioxygen are, at least in part, due to peculiar differences of the deoxy-forms. The peroxide ligand in oxy-Hc represents a structural constraint that normalizes the metal-ligand complex in the various Hcs. Molecular heterogeneity and sovramolecular organization of hemocyanins and hemoglobins Hemocyanins (Hcs) and invertebrate hemoglobins (Hbs) (including chlorocruorins) are giant molecules. The aggregates that are found in vivo exhibit molecular weights ranging between 450 kDa and 9.0 MDa according to the species. Such proteins have a very peculiar quaternary structure that can be described on the basis of relatively simple models. Thus, molluscan Hcs are referred to as hollow cylinders with a 5- or 10- fold symmetry axis. In cephalopods, the decamers are the only hemocyanin quaternary structure observed, but in gastropods two decamers are assembled face-to-face to form the so-called di-decamer. The diameter of these molecules is 35 nm and the height is 36 nm in gastropods and 18 nm in cephalopods. Upon increasing the pH in absence of divalent cations all molluscan Hcs dissociate in subunits with sedimentation coefficient 11S and molecular weight range between 250 and 450 kDa according to the species. Such subunits are folded as to form several globular functional units (FUs), with a molecular mass of 45-65 kDa and each containing one active site. The structure at 15 Å resolution of the di-decamer of keyhole limpet hemocyanin isoform KLH1, the 2.3-Å resolution of the tertiary structure of the C-terminal FU of Octopus dofleini Hc, the wealth of data from immunoelectron microscopy, the 12-Å reconstruction of the quaternary structure of Haliotis tuberculata Hc and recent hypothesis for evolution of the quaternary structure of molluscan Hcs, have increased the information about the exact topological position of the various FUs types and the path of the subunits to form the hollow cylinders. In a number of studies carried out at the CNR Centre we focused on the quaternary organization of molluscan Hcs using two different approaches. The first one consisted in the kinetic and equilibrium study of molluscan Hc dissociation, in order to obtain information on the hierarchical organization of the native molecules. For these goals, light scattering stopped-flow -for kinetic measurements-, electron microscopy with negative staining and small angle X-ray absorption techniques (SAXS) -for equilibrium measurements- has been applied. The latter techniques have been conveniently implemented with image reconstruction methods and structural analysis software for defining a structural model of the macromolecule. In particular, a technique for reconstructing iceembedded macromolecules from electron micrographs taken at two specimen tilts (+/-23 degrees) has been used to determine the structure of chlorocruorin isolated from the Polychaete annelid Sabella spallanzanii. The structure of this protein, essentially the same as that for Lumbricus terrestris is a bilayer structure with overall symmetry D6, containing six hollow groups per layer. A hollow group is formed by six globular masses and has approximate threefold symmetry. Other structural elements connect the two layers and the hollow groups in a layer. This non-globin material occupies about 15% of the total molecular volume. The elaboration of SAXS data is still in progress for both Hbs and Hcs and ultimately will allow for: the specification of the position of each structural subunit within the oligomer (Hbs and Hcs), the role of water-protein interaction for the conformational transition between the two functional states of the protein: oxy- and deoxy-Hc. Such kind of analysis has been made very reliable in the recent years by the development of robust simulation algorithms that can approach the problem starting from the crystallographic coordinates of the subunit or FU. The second approach consists in the deconstruction of the native molecule to reduce complexity. Then, the products to be studied are the structural subunits or the FUs that can be produced by chemical cleavage of covalent bonds linking them together in the structural subunit. When the structural changes at the active site levels, depending on oxygen coordination, could be related to the structural changes at the dimensional scale of the whole molecule we will be able to trace the conformational demands that define the allosteric properties of the oligomer. Furthermore the elucidation of the sequence of subunits or of FUs will allow to trace the evolutionary pathways within each protein type. The native form of hemocyanin (Hc) from Octopus vulgaris can be completely dissociated, at alkaline pH and in the presence of EDTA, from 49S decamers to 11S monomers. The kinetics of this process shows that the dissociation of decamers to monomers takes place in three consecutive and irreversible steps, with a highly cooperative step concerning dissociation of octamers to dimers, which appears to be the only intermediate species. From such structural units, the FUs can be obtained, on the basis of literature methods, by limited proteolysis using suitable proteolytic enzymes. The current literature model for molluscan Hcs involve the presence, within the structural subunit, of globular FUs, constituted about 400 amino acid residues that are connected by portions of the polypeptide chain, consisting of 10-15 amino acid residues, more sensible to proteases than the globular regions. The FUs are identified a-h, starting from the amino terminus of the subunits. 20 Limited proteolysis with -chimotrypsin of native Rapana venosa hemocyanin allows the separation of three functional proteolytic fragments with molecular mass of 150, 100 and 50 kDa. The functional fragments, purified by combining gel filtration and ionic exchange FPLCromatography, were analysed by means of small angle X-ray scattering (SAXS). Independent shape determination of the 50 kDa and 100 kDa proteolytic fragments yields consistent low-resolution models. A simultaneous fitting of the SAXS data from these fragments provides a higher resolution model of the 100 kDa species made of two functional units tilted with respect to each other. The model of the 150 kDa proteolytic fragment consistent with the SAXS data displays a linear chain-like aggregation of the 50 kDa functional units. These observation provide valuable information for the reconstruction of the three-dimensional structure of the minimal functional subunit of gastropod hemocyanin in solution. Furthermore, the spatial relationships among the different functional units within the subunit will help the elucidation of the overall quaternary structure of the oligomeric native protein. We have recently observed that by Zn2+ treatment of the 11S structural subunit it is possible to obtain with high yield 50 kDa fragments that are currently under investigation to compare their N-terminal sequences with those of the FUs produced by proteolytic cleavage with the ultimate goal to identify the cleavage site and the reaction mechanism. As outlined above, arthropod Hcs are refeered to a completely different organization plan. Native arthropod Hcs are multimeric proteins where each subunit has a molecular mass of about 75 kDa (5S) and contains one binuclear copper site capable of oxygen binding. Different functional subunits are arranged as hexamers (16S corresponding to 16-mer or building block) or multiples of hexamers (24S, 36S, 48S and 62S corresponding to 2x6-mers, 4x6-mers, 6x6-mers and 8x6-mers, respectively). The native aggregation level appears to be characteristic for each species and may also be related to the taxonomic groups. In the hemolymph of most crustacea, hexamers (16S) or dodecamers (24S) or both are found. However, the biological significance of the different aggregation states is still controversial. Although most arthropod Hcs exhibit only one or two aggregation states in vivo, it is usually possible to obtain dissociation to the lower structure by a proper choice of non-physiological solution conditions. In particular, removal of divalent cations (Ca ++, Mg++) by dialysis against EDTA and/or a change of the pH value from the neutral range will cause dissociation, which in many cases is reversible. An interesting feature of the arthropod Hcs is their marked heterogeneity at the subunit level. Such heterogeneity derives from both differences in amino acid composition and molecular weights. These types of differences have been observed in all arthropod Hcs with a possible exception for the polypeptide chain composition of the 16S hexameric components of some isopods. Generally the assembly of the higher di- tetrahexa- and octa-hexameric hierarchies found in various crustacean, chelicerate and centipede Hcs, requires a large number of different subunits. In fact, while a single type of subunit appears to be adequate for the correct reassembly of the basic hexameric unit, certain key bridging or “linker” subunits are necessary for the formation of higher assemblies, larger than hexamers. Different subunits occupy specific locations within the oligomeric structure and are generally present in constant proportions. Furthermore, the total set of subunits is required to reorganize the original aggregate from a mixture of dissociated products, indicating that each of them plays a specific role in the architecture of the protein. This is indicated by the fact, that purified subunits often possess the ability to assemble into homohexamers, but heterogeneity is necessary for the correct assembly of the native quaternary structure. Subunit heterogeneity has functional role in the modulation of the oxygen binding properties of Hcs; in particular, it seems to be required for the adaptation of organisms in response to changes of the environmental conditions. There is evidence that in some Crustacea Hc, the subunit composition varies with season, oxygen level, or salinity, which results in a modulation of the functional properties. Furthermore, it has been shown that the polymorphism is not genetically fixed and, in relation to this, changes in structural and functional phenotypes of Hc in response to specific environmental stimuli have been observed. The oxygen binding properties of arthropod Hcs have a significant dependence on pH, concentrations of specific ions and other allosteric effectors. The p50 values vary widely, reflecting the wide variety of environmental conditions and behavioural patterns observed for different organisms. The degree of cooperativity for oxygen binding also shows a wide range of variation and is generally greater in Hcs containing larger number of subunits. On these grounds we have studied the molecular heterogeneity of a number of crustacean Hcs isolated from species that inhabit peculiar areas or exhibit peculiar physiological responses to the environmental stimuli. The strategy behind these studies is to characterize the subunit pattern of a given crustacean Hc and to correlate it with the allosteric properties exhibited in oxygen binding. This approach provides the information necessary to evaluate the changes at molecular level that are induced from a modification of environmental parameters. As described above for Hcs, also the annelid Hbs are organized in a very complex sovramolecular network of interacting polypeptides, the structure of which is still not wholly resolved. We were able to separate by twodimensional electrophoresis the 4-MDa chlorocruorin of Sabella spallanzanii and identify its components by amino-terminal sequencing. This approach revealed a high heterogeneity of constituent chains in a single animal as well as in the Sabella population. Using a cDNA library prepared from the haematopoietic tissue of this worm, 21 we have isolated and fully sequenced most globin and linker cDNAs. The primary structure features of these polypeptides have been characterized by comparison with model globin and linker sequences. The evolution of extracellular hemoglobins of annelids, vestimentiferans, and pogonophorans was then investigated by applying cladistic and distance-based approaches to reconstruct the phylogenetic relationships of this group of respiratory pigments. We performed this study using the aligned sequences of globin and linker chains that are the constituents of these complex molecules. Our results allowed us to test previous hypotheses on the evolutionary pathways of these proteins and to formulate a new most parsimonious model of molecular evolution. According to this novel model, the genes coding for the polypeptides forming these composite molecules were already present in the common ancestor of annelids, vestimentiferans, and pogonophorans. 22 4. PHOTOSENSITIZING AND PHOTOTHERAPEUTIC PROPERTIES OF PORPHYRINS AND THEIR ANALOGUES The phototoactivation of porphyrins and their analogues, which posses a tetrapyrrolic macrocycle (chlorins, porphycenes, purpurins, phthalocyanines and naphthalocyanines), is a research area of increasing interest. The photobiological properties of such compounds are the basis for the development of a variety of applications ranging from basic research to biotechnologies. Such applications include: the conversion of solar energy into chemical energy by artificial systems mimicking the natural photosynthetic apparatus; mapping the microenvironment of porphyrin-type prosthetic groups in proteins the controlled generation of reactive oxygen species with an aim to study their physico-chemical properties in homogeneous and microheterogeneous biological systems; the promotion of photobiostimulation and photosignalling processes starting from predetermined studies where the incident photons are piloted, e.g. the onset of apoptotic processes, the induction of focalized mutations and the activation of specific enzymic functions; the development of phototherapeutic techniques, mainly in oncology (photodynamic therapy of tumours), as well as in dermatology (phototherapy of psoriasis and actinic keratosis), atherosclerosis (prevention of artery restenosis after balloon angioplasty), and the sterilization of viral and microbial infections. This wide range of applications is related with the possibility to chemically synthesize porphyrin derivatives with a fine-tuned chemical structure; this allows the modulation of their physico-chemical properties and their distribution pattern at a cell/tissue level. The importance of such modulation arises from the short lifetime of the photogenerated reactive species, so that the porphyrin-promoted photoprocesses take place in a restricted (a few nm) spatial range around the porphyrin binding site. The variation of the chemical structure of porphyrins is achieved through the control of: the extension of the tetrapyrrolic macrocycle; the nature of the peripheral substituents; the presence of metal ions coordinated with the pyrrolic nitrogens; the nature of the axial ligands to the metal ions. In general, our research line is developed according to the following steps: a. chemical synthesis of porphyrins with predetermined chemical structure b. spectroscopic characterization of the porphyrins in their ground and electronically excited states c. determination of the quantum yield of the photogeneration of reactive intermediates d. photokinetic study of the photosensitized modification of model biomolecules (e.g. steroids, unsaturated lipids, aromatic amino acids) e. affinity of individual porphyrins for non-transformed, tumour, microbial cells and determination of dark cytotoxicity and subcellular distribution their f. kinetics and mechanism of porphyrin-sensitized cell photoinactivation and correlation with the subcellular distribution. g. pharmacokinetic and photosensitizing properties of porphyrins in vivo h. applications of porphyrin-photosensitised processes for the photodynamic therapy of tumours and other diseases 23 Main Results a) Synthesis of Porphyrinoids As mentioned in the introductory paragraph several approaches are available in order to engineer porphyrin molecules with different chemical structure, hence with predetermined physico-chemical properties. In our investigations we were mostly interested in porphyrin derivatives having an extended aromatic conjugation in order to shift the absorption spectrum toward the far-red/near-IR wavelength region which is of high interest for medical applications. Thus, we prepared the following porphyrinoids with an extended macrocycle: porphycenes, which represent electronic isomers of porphyrins with a 18 electron cloud; chlorines, which are characterized by the reduction via hydrogenation of one pyrrole ring; phthalocyanines and naphthalocyanines, where a benzene and, respectively, a naphthalene ring is condensed with each pyrrole moiety. Moreover, for each class of compounds, we used both the free base and selected metallo-derivatives: several metal ions can be coordinated at the centre of the macrocycle, however only diamagnetic ions are of interest for photobiological applications; therefore, we prepared porphyrinoid derivatives with Mg(II), Zn(II), Al(III), Ge(IV), Si(IV) and Sn(IV). In a few cases, axial ligands were placed in the fifth and sixth coordination positions of the central metal ion as an additional tool to modulate the physico-chemical and (photo)biological features of the porphyrinoid molecules. Lastly, we focused our attention on porphyrinoids substituted in the peripheral positions of the macrocycle by functional groups with well defined chemical structure, in order to generate compounds differing in size, electric charge, hydrophilic or hydrophobic character, and bulkiness. Typical examples are represented by polycarboxylated or poly-sulphonated derivatives, alcoholic groups linked to the pyrroles via hydrocarbon chains of different length, aliphatic amino groups or pyridine/piperidine rings with both tertiary or quaternary nitrogen atoms, etc. All the compounds were analysed for their chemical purity by thin layer chromatography or HPLC techniques, as well as characterized by absorption and NMR spectroscopy. The various derivatives were also studied for their chemical stability in the dark and under visible light-illumination both in the solid state and in solution. In general, porphyrins and their analogues display an excellent chemical stability and a longer shelf life. b) Spectroscopic characterization As a rule the porphyrin derivatives synthesized by us displayed absorption bands peaking at longer wavelengths and possessing larger molar extinction coefficients as compared with porphyrins. Such spectroscopic properties were important since they guaranteed a higher probability and efficiency of light absorption, hence of generation of electronically excited states. The absorption spectra of the various porphyrins were monitored as a function of the porphyrin concentration in order to define the molarity range where the Beer-Lambert law was strictly followed, thus allowing us to identify the conditions corresponding to a purely monomeric state of the porphyrin; in actual fact, as outlined below, aggregated porphyrins are most frequently characterized by a poor photosensitising activity. The aggregation of porphyrins, which reflects the tendency of the flat tetrapyrrolic macrocycle to yield face-toface dimers or higher oligomers due to hydrophobic interactions, is more pronounced in aqueous media, while its importance decreases upon moving to organic solvents or micellar/liposomal dispersions of surfactants and phospholipids. Porphyrins undergo a ready partitioning into the non polar section of micelles and liposomes. The absorption spectroscopic studies were integrated by fluorescence excitation and emission measurements, which were performed under both steady-state and time-resolved conditions. Such measurements would yield important information on two main aspects: 1) The quantum yield of fluorescence emission, which indicates the probability of radiative decay of the porphyrins from the initially formed lowest excited singlet state; such emission is often used in biological systems for a quantitative determination of the porphyrin concentration, as well as for probing the nature of porphyrin microenvironment and binding site in a cell or tissue. 2) The presence of multiple porphyrin species, for example a mixture of monomeric and aggregated porphyrins: the porphyrin aggregates generally exhibit a reduced fluorescence quantum yield, and a shorter emission lifetime; if the fluorescence decay is monitored according to a time-resolved regime, one can obtain a quantitative measurement of the relative weight of each porphyrin species. Determining the amount of aggregated species has also a predictive role for the photosensitising efficiency of porphyrins. In general, the quantum yield of porphyrin fluorescence emission does not exceed 0.2 and in many cases is below 0.1. This value points out that a predominant fraction of the electronically excited porphyrin molecules undergo intersystem crossing to the lowest excited triplet state: the latter plays a major role in porphyrin-photosensitized processes owing to its particularly long lifetime (of the order of milliseconds) even in fluid media. 24 c) Photogeneration of reactive intermediates As mentioned in the previous paragraph, the lowest excited triplet state represents the main reactive intermediate in photosensitised processes promoted by porphyrins and their anlogues. The efficiency for the generation of such electronically excited state can be quantitatively measured through a specific parameter, the quantum yield, which is the ratio between the number of triplet species formed vs. the total number of photoexcited photosensitiser molecules. This parameter can be obtained by means of laser flash photolysis techniques with a resolution level of microseconds. In general, both free base and metallo-porphyrinoids coordinated with diamagnetic metal ions exhibit quantum yields for triplet photogeneration higher than 0.5; such a value underlines the appreciable overall photosensitising efficiency of these tetrapyrrolic derivatives. The lifetime of porphyrin triplet states is in the millisecond range, which gives these species a high probability to diffuse over relatively large distances in fluid media. In actual fact, two reaction pathways are open to triplet porphyrins: 1) the direct interaction with a nearby substrate molecule with formation of radical derivatives, which in turn react with oxygen to yield oxidized products; 2) energy transfer to oxygen with promotion of the latter to singlet oxygen, a hyper-reactive and highly cytotoxic species. While in principle both mechanisms occur in porphyrin-sensitised photoprocesses, the singlet oxygen pathway usually predominates especially in hydrophobic media (e.g. organic solvents of low dielectric constant, internal regions of globular protein molecules, lipid domains of cell membranes) where the oxygen concentration is particularly large and the singlet oxygen lifetime is longer. This feature allows one to classify porphyrinoid compounds as typical “photodynamic” sensitisers (i.e. oxygen-requiring photosensitisers). Two factors may inhibit the photosensitising action of porphyrins, namely (1) the formation of aggregated species (which most frequently occurs in aqueous media) owing to the related drop in the lifetime of the triplet state; and (2) the insertion of paramagnetic metal ions (e.g., Fe (III), Co(III) ions) at the centre of the macrocycle: such derivatives posses a quantum yield of triplet generation close to unity, however the triplet leifetime falls below the nanosecond level thereby preventing any interaction between the triplet and other molecules. Lastly, we ascertained that photoexcited porphyrins may also generate syperoxide anion via electron transfer to oxygen. However, the efficiency of this process is about 1% that typical of singlet oxygen generation,hence superoxide hardly plays any important role in porphyrin-photosensitised processes. d) Photosensitised modification of model biomolecules Preliminary studies demonstrated that the following biomolecules are most readily susceptible to photooxidative attack sensitised by porphyrins and their analogues: - Aromatic and sulfur-containing amino acids, such as tryptophan, histidine, tyrosine, cysteine and methionine. - Unsaturated lipids and steroids, including cholesterol - Guanosine nucleotides On these bases we selected both tryptophan, in the form of N-acetyl-L-tryptophanamide (NATA) and cholesterol as model substrates for testing the photosensitising efficiency of the various porphyrinoids. In all cases, the two substrates were photomodified according to first order kinetics: the efficiency of the photoprocesses can be determined via the frist order rate constant of the semilog plot: [substrate] vs. irradiation time. Tryptophan photooxidation, leading to the formation of an oxidised derivative, namely Nformyl-kynurenine, occurred with k = 105 s-1, which is in line with highly efficient photodynamic sensitisers. This conclusion was further supported by the data on cholesterol photooxidative conversion to its 5- and 7hydroperoxides, which occurred with k = 104 s-1. As expected, such photoprocesses were found to be most efficient in lipophilic microenvironments: typical examples are represented by liposomal vesicles where the porphyrinoid molecules are embedded in the phospholipid bilayer or non-covalent complexes between the porhyrinoids and lipoproteins where the porphyrin is partitioned within the large lipid moiety. e) Binding of porphyrins to eukaryotic and prokaryotic cells The affinity of porphyrinoids for essentially all types of cells is strongly influenced by their chemical structure. Thus, in the case of mammalian cells, including normal and transformed fibroblasts, amelanotic and melanotic melanoma cells and keratinocytes, maximum accumulation was obtained by using lipophilic and amphiphilic derivatives: for example, porphyrins bearing two to eight phenyl rings or ester functions as peripheral substituents, or porphyrins bearing two carboxylate groups on adjacent pyrrole rings. In general, the kinetics of porphyrin uptake by mammalian cells is rather slow, with significant endocellular concentrations reached after 23 h incubation. The incubation time also affects the pattern of subcellular distribution of the porhyrins: thus, the cytoplasmic membrane represents the most important binding site for short (no longer than 1-3 h) incubations; prolonging the 25 incubation time favours a redistribution of the porphyrins to endocellular membranous compartments, including mitochondria, lysosomes, the Golgi apparatus and the rough endoplasmic reticulum. This pattern is also dependent on the delivery system adopted for the administration of hydrophobic porphyrinoids to cell cultures: thus, preincorporation of the porphyrins in liposomes or low-density lipoproteins (LDL) promotes endocytotic processes with a release of the photosensitiser to endocellular sites. The porphyrin delivery via LDL is of particular interest in the case of tumour or other kinds of rapidly proliferating cells, which are usually characterized by an overexpression of LDL-specific receptors; as a consequence, the administration of the porphyrin by this route leads to a selective or at least preferential accumulation of the photosensitiser in neoplastic as compared with normal cells. An essentially identical picture was obtained upon studying the accumulation and subcellular partitioning of porphyrins in yeast cells, such as Candida albicans, as well as in prokaryotic cells, such as Gram-positive bacteria. It was found that amphiphilic porphyrins readily cross the outer wall surrounding the plasma membrane and localise in large amounts at the level of specific protein constituents of the membrane. the rate and extent of porphyrin accumulation can be modulated to some degree by controlling the amount of cholesterol in the membrane, Increasing such paarmeter causes an increased membrane rigidity and a less pronounced overall uptake of the porphyrinoid molecules. Thus, the cytoplasmic membrane again appears to represent the main binding site in such cells. A somewhat different picture was observed in the case of Gram-negative bacteria: these cells are characterized by the presence of a highly organized outer wall, which can efficiently intercept the externally added photosensitiser molecules. Only upon enhancing the permeability of the wall was a signifcant amount of porphyrinods accumulated at the level of the plasma membrane in typical Gram-negative bacterial strains, such as Escherichia coli or Pseudomonas aeruginosa. The permeabilization was obtained by addition of EDTA, which chelates some Ca2+ counterions neutralizing the negatively charged groups at the surface of the outer wall of these bacteria, or peptide-type agents, such as polymixin or polylysine. Quite interestingly, no difference in the rate and extent of porphyrinoid accumulation was detected between wild-type and antibiotic-resistant strains, including methycillin- and vancomycin-resistant strains of Staphylocccus aureus or multidrug resistant Pseudomonas aeruginosa. This observation opens the way to the use of photodynamic processes as a tool for the inactivation of antibiotic-resistant microbial pathogens. In no case, detectable amounts of porphyrinoid derivatives were found to bind with the DNA or other genetic material in both prokaryotic and eukaryotic cells. As discussed more in detail in a later paragraph, this circumstance decreases the probability that nuclear damage plays a major role in photosensitised inactivation of cells, thereby minimizing the risk of inducing the onset of mutagenic or cancerogenic processes in partially inactivated cells. Porphyrin-loaded mammalian and bacterial cells underwent no detectable toxic effect when incubated in the presence of porphyrin concentrations lower than 10 M; such concentrations are most efficient in inducing photochemical damage to cells. Dark toxicity is only observed for porphyrinoid concentrations larger than 25-30 M. Therefore, a suitable choice of the experimental parameters, such as the porphyrinoid dose and the preirradiation incubation time, allows one to avoid any generalized toxic effects and focus the photosensitised process within the illuminated area. This feature represents the basis for the application of porphyrinoidphotosensitised processes in medical fields, as discussed below. f) Porphyrin-photosensitised inactivation of mammalian and microbial cells Irradiation of porphyrinoid-loaded cells with light specifically absorbed by the photosensitising agent invariably results in the damage of subcellular sites surrounding the porphyrinoid binding sites. The overall photoprocess is characterized by a high degree of selecitivity owing to the very short lifetime and high reactivity of the phototoxic intermediate species. As a consequence, in the case of mammalian cells, the most readily damaged sites are represented by the cell membranes in agreement with the above detailed pattern of subcellular distribution typical of porphyrins and their analogues. In particular, the photodamage is restricted within the plasma membrane for irradiations performed after relatively short incubation times (1-3 h), while as the incubation is prolonged inner membranous districts are more heavily involved in the photoprocess. In this connection, some “fine tuning” of the photodamaged sites can be achieved by selecting the chemical structure of the porphyrinoid photosensitiser: thus, cationic derivatives preferentially affect the mitochondrial membrane, while hydrophobic (liposome-delivered) porphyrins largely affect the lysosomal bag. The photosensitised and photodamaged cells exhibit some tendency to promote repair processes, largely through the expression of stress proteins. However, in most cases, cell death represents the obvious consequence of the photoprocess: the death can occur via either random necrosis or apoptosis; the relative weight of the two modalities of cell death is controlled by a variety of factors, whose precise role has not yet been determined. Present evidence suggests that apoptosis is favoured by mild irradiation conditions and by the use of mitochondria-bound porphyrins which induce the inactivation of the antiapoptotic protein Bcl-2. 26 Depending on the distribution pattern of the porphyrinoid photosensitiser, the biological consequences of the photoprocess include the impairment of transmembrane transport of metabolites, drop in specific enzymic activites, alteration of membrane potential, cross-linking of membrane or cytoplasmic proteins etc. In any case, nuclear damage takes place only after prolonged irradiation and is not correlated with cell death. The latter event is rather a consequence of osmotic unbalance and inhibition of ATP synthesis. As regards the microbial cells, an extensive (5-6 log) decrease in the population of living cells is generally obtained by using markedly milder irradiation conditions as compared with mammalian cells: thus, photosensitised killing of such cells is very effective already after 1 min incubation with porphyrinoid concentrations as low as 0.1 M, which are ineffective toward mammalian cells. This feature is extremely important for possible phototherapeutic applications of these photoprocesses to treat microbial infections, since it guarantees a high degree of selectivity toward the microbial pathogens in comparison with host tissues. Once again, the photoinduced damage is largely limited to the plasma membrane with no significant involvement of the genetic material: no evidence for mutagenicity or repair of the photodamage has been obtained with all the bacterial and yeast strains investigated by us. g) Pharmacokinetic and photosensitisation studies in experimental animals The possibility to use porphyrin-photosensitized processes for the so called photodynamic therapy (PDT) of tumours was preliminarily investigated in tumour-bearing mice. Two main tumour models were used, namely MS-2 fibrosarcoma intramuscularly transplanted in Balb/c mice and B16 melanotic melanoma subcutaneously transplanted in C57 mice. The use of PDT is based on the assumption that the photosensitising agent is selectively or preferentially accumulated in the neoplastic lesion so that the photodamage induced by irradiation with light wavelengths specifically absorbed by the photosensitizer is confined in the diseased tissue. To achieve this goal the porphyrins or their derivatives need to be transported by serum LDL , which express a preferential interaction with tumours cells via receptor-mediated endocytosis. The porphyrins with higher affinity for tumours are actually characterized by a large degree of hydrophobicity: the latter parameter is measured in terms of partition coefficient of the photosensitizer between n-octanol and water; partition values higher than about 10 are typical of tumour-selective porphyrins. The porphyrins are intravenously administered after incorporation into liposomal vesicles, which are engineered in such way to promote their extremely rapid fusion with LDL, thereby releasing the photosensitizer to the lipoprotein. A rigorous control of the transport modality of the systemically injected porphyrin yields photosensitizer concentration ratios as high as 20 between the tumour and the peritumoural districts. In general, the maximum concentration of porphyrin-type photosensitizers in the tumour tissues is reached within 24 h from injection; however, the clearance form the tumour is generally slow so that significant concentrations of hydrophobic porphyrins in the neoplastic tissue are preserved for at least 72 h. The porphyrin molecules localize inside the neoplastic cells with only minor concentrations in the neovascular network. As a consequence, upon irradiation of the tumour area, a direct killing of neoplastic cells is obtained with only minor vascular damage; this ensures a sufficiently large supply of oxygen to the irradiated tissue, minimizing the risk of formation of hypoxic clusters, from which recurrencies most frequently originate. The irreversible damage of the tumour takes place by both random necrotic and apoptotic pathways, analogously with what observed for photosensitised cells. While the fibrosarcoma is efficiently phototreated by using both porphyrins and their farred absorbing analogues (chlorins, phthalocyanines), the pigmented melanoma can be successfully photosensitised only by using compounds, such as naphthalocyanines, which exhibit intense absorption bands in the wavelength region above 800 nm, where the residual absorbance by melanin is of minor importance. The understanding of the modalities by which porphyrinoids are transported in the bloodstream, interact with rapidly proliferating or normal cells, and photosensitize cell damage opened the way to the PDT of diseases which are outside the oncological field. This area of research is steadily expanding. In our laboratory we started two new phototherapeutic applications: 1. The treatment of occluded arteries after balloon angioplasty in order to prevent the occurrence of restenosis. This technique involves the local delivery of the photosensitizer in the lesioned arterial segment followed by irradiation with specific light wavelengths, which are piloted to the irradiation site via optical fibres connected with a laser source. So far a preclinical PDT protocol has been developed through studies carried out on rabbits whose arteries were opened by angioplasty and subsequently subjected to photosensitization. The best results were obtained by using a Zn(II)-phthalocyanine delivered via a specifically built catheter: the photoprocess brings about a selective destruction of smooth muscle cells, which are usually responsible for the restenosis. The photodamage is confined to the intimal layers with no appreciable involvement of the media and adventitia. 2. The treatment of microbial infections, especially those chronicized after antibiotic therapy. As a matter of fact, porphyrinoid photosensitizers are just about as active against normal and antibiotic-resistant microbial strains. Once again the most promising results have been obtained by using phthalocyanine-type photosensitizers, which promote the photoinactivation of Gram(+) and Gram(-) bacteria, yeasts, fungi and 27 micoplasmas by using just one mild irradiation protocol. In vivo studies with artificially infected mice or spontaneously infected dogs and cats clearly indicate that an extensive decrease in the microbial population can be achieved with a minimal parallel damage to different types of host tissues. h) Phototherapeutic applications at a clinical level The experience accumulated through years of research activity in the field of PDT allowed us to establish active and continuative collaborations with selected clinical centres for the treatment of specific diseases. Our group is involved in monitoring the pharmacokinetic behaviour of the systemically injected or topically administered photosensitizers, the definition of the clearance rate from blood and diseased tissues, the validation of any variations in the standard clinical protocols. In particular, our activity is focused in the PDT of the following pathologies: - solid tumours of the breast, brain and skin (particularly successful is the treatment of AIDS-related Kaposi sarcoma) - recurrencies from surgically or radiotherapeutically treated melanotic melanoma - infections of skin and burns caused by Staphylococcus aureus , including its methicillin-resistant strain - stimulation of wound healing by sterilization of the intralesional bacterial flora - treatment of periodontal and other oral cavity diseases caused by Candida albicans or bacterial agents. 28 PUBLICATIONS (1970 – 2001) (1970) ALBERGONI, V., CASSINI, A., SALVATO, B. Ricerche sulle Emocianine. XIII. Isolamento e analisi di alcune frazioni glicopeptidiche dell`emocianina di Octopus vulgaris. Boll. Soc. Ital. Biol. Sper., 46, 939 (1970). SALVATO, B., GHIRETTI-MAGALDI, A., ALBERGONI, V. Ricerche le Emocianine. XIV. Terminali amminici delle emocianine di Octopus vulgaris e di Carcinus maenas. Boll. Soc. Ital. Biol. Sper., 46, 943 (1970). SALVATO, B., GHIRETTI-MAGALDI, A., TALLANDINI, L. Ricerche sulle Emocianine. XV. Titolazione acido-base dell’emocianina di Octopus vulgaris. Boll. Soc. Ital. Biol. Sper., 46, 961-964 (1970). (1971) SALVATO, B., SARTORE, S., ZACCARIA, C., GHIRETTI-MAGALDI, A. Ricerche sulle emocianine. XIX. Peso molecolare delle subunità funzionali e delle catene polipeptidiche delle emocianine di Octopus vulgaris. Boll. Soc. Ital. Biol. Sper., 47, 777 (1971). SALVATO, B., TALLANDINI, L., GHIRETTI-MAGALDI, A., GHIRETTI, F. Ricerche sulle emocianine. XVII. Variazioni conformazionali dell’emocianina di Octopus vulgaris. Boll. Soc. Ital. Biol. Sper., 47, 773-774 (1971). SALVATO, B., TALLANDINI, L., GHIRETTI-MAGALDI, A., GHIRETTI, F. Ricerche sulle emocianine. XVIII. Titolazione spettrofotometrica delle tirosine nell’emocianina di Octopus vulgaris in presenza di tiourea e di tiocianato. Boll. Soc. Ital. Biol. Sper., 47, 775-776 (1971). (1972) ALBERGONI, V., CASSINI, A., SALVATO, B. The carbohydrate portions of hemocyanin from Octopus vulgaris. Comp. Biochem. Physiol., 418, 445 (1972). GHIRETTI, F., SALVATO, B., CARLUCCI, S., DE PIERI, R. Manganese in Pinna nobilis. Experientia, 28, 232(1972). SALVATO, B. Ricerche sulle Emocianine.XXII. Perfezionamento del metodo ditisone per la determinazione del rame. Boll. Soc. Ital. Biol. Sper., 48, 1314 (1972). SALVATO, B., SARTORE, S., RIZZOTTI, N., GHIRETTI-MAGALDI, A. Molecular weight determination of polypeptide chains of molluscan and arthropod hemocyanins. FEBS Letters, 22, 5 (1972). SALVATO B., GHIRETTI-MAGALDI A., ZATTA P., GHIRETTI F. Ricerche sulle Emocianine. XXI. Titolazione acido-base della emocianina di Octopus vulgaris in soluzione di 3urea e guanidina. Boll. Soc. Ital. Biol. Sper., 48, 1312 (1972). SALVATO, B., ZATTA, P. Ricerche sulle Emocianine. XX. Esclusione degli -NH2 terminali dal sito attivo dell`emocianina. Boll. Soc. Ital. Biol. Sper., 48, 1311 (1972). (1973) GHIRETTI, F., GHIRETTI-MAGALDI, A., SALVATO, B. The chemical basis for the evolution of hemocyanins. In: Comparative Physiology (Bolis, L. et al., eds.) North Holland Pbublishing, Amsterdam, p. 509 (1973). SALVATO, B. Ricerche sulle emocianine. XXII: Perfezionamento del metodo dei ditizone per la determinazione dei rame. Boll. Soc. Ital. Biol. Sper., 48, 1315 (1973). GHIRETTI-MAGALDI, A., GHIRETTI, F., SALVATO, B. The chemical basis of Hemocyanins. 29 In: Comparative Physiology. (L.Bolis et al. Eds.) 1973, p. 509. GHIRETTI, F., GHIRETTI-MAGALDI, A. Respiratory proteins in Molluscs. In: Chemical Zoology(Florkin,M.,ed.) Acad. Press,Vol.VII,p.201 (1973). GHIRETTI-MAGALDI, A., MILANESI, C., SALVATO, B. Ricerche sulle emocianine. XXIII: I cianoblasti e i cianociti nei tessuti emopoietici di Carcinus maenas. Boll. Soc. It. Biol. Sper., 48, 1316 (1973). GHIRETTI-MAGALDI, A., MILANESI, C., SALVATO, B. Ricerche sulle emocianine. XXIV: Identificazione della proteina nei cianoblasti e nei cianociti di Carcinus maenas. Boll. Soc. It. Biol. Sper., 48, 1318 (1973). GHIRETTI-MAGALDI, A., MILANESI, C., SALVATO, B. Identification of hemocyanin in the cyanocytes of Carcinus maenas. Experientia, 29, 1265 (1973). SALVATO, B. Ricerche sulle emocianine. XXII: Perfezionamento del metodo dei ditizone per la determinazione dei rame. Boll. Soc. Ital. Biol. Sper., 48, 1315 (1973). SALVATO, B., ZATTA, P., GHIRETTI-MAGALDI, A., GHIRETTI, F. On the active site of Hemocyanin. FEBS Lett., 32, 35 (1973). SALVATO, B., TAMBURRO, G.A., GHIRETTI-MAGALDI, A. On the active site of Hemocyanins. IX Intern. Congress Biochemistry, Stockholm, p. 112, 2sll (1973). SALVATO, B., ZATTA, P. Ricerche sulle emocianine. XX:Esclusione dei gruppi NH2 dal sito attivo dell’emocianina di Octopus vulgaris. Boll. Soc. Ital. Biol. Sper., 48, 1311 (1973). SALVATO, B., ZATTA, P., GHIRETTI-MAGALDI, A., GHIRETTI, F. Ricerche sulle emocianine. XX: Titolazione acido-base dell’emocianina di Octopus vulgaris in presenza di urea e guanidina. Boll. Soc. Ital. Biol. Sper., 48, 1312 (1973). (1974) ALBERGONI, V. Ricerche sul metabolismo dei rame. Arch. Fisiol.,71, 1 (1974). ALBERGONI, V., CASSINI, A. A cupro-zinc protein with superoxide dismutase activity from liver. Comp. Biochem. Physiol., 47B, 767 (1974). ALBERGONI, V., COBELLI, C., FRANCINI, G.L. Biological Systems. Pitagora Editrice, Bologna (1974). FINAZZI-AGRO’, A., ALBERGONI, V., CASSINI, A. Intrinsic fluorescence of a protein devoid of tyrosirie and tryptophan: horse hepatocuprein. FEBS Lett., 39, 164 (1974). GHIRETTI, F. L’evoluzione a livello molecolare. In: Le basi biologiche della Medicina moderna, Edizioni Medico Scientifiche, Torino, Vol. 1, p. 215 (1974). SALVATO, B., GHIRETTI-MAGALDI, A., GHIRETTI, F. Acid-base titration of the hemocyanin from Octopus vulgaris. Biochemistry, 13, 4778 (1974). (1975) ALBERGONI, V., CASSINI, A. Superoxide-dismutase in Euglena gracilis. Gior. Bot. Ital., 109, 160 (1975). ALBERGONI, V., CASSINI, A. The oxidation of cysteine by ceruloplasmin. 30 FEBS Lett., 55, 261 (1975). ALBERGONI, V., CASSINI, A., FAVERO, N., ROCCO, G.P. Effect of penicillamine on some metals and metallo—proteins in the rat. Biochem. Pharmacol., 24, 1131 (1975). GHIRETTI, F., GHIRETTI-MAGALDI, A. Respiration. In: Pulmonates (Fretter, ed.) Vol. I, Acad. Press (1975). GHIRETTI-MAGALDI, A. Biosintesi in vivo dell’emocianina. Identificazione dei cianoblasti e dei cianociti in Carcinus maenas. Atti V Congr. Soc. It. Biologia Marina, Tip. Ed. Salentina (1975). JORI, C. Photosensitized reactions of’amino acids and proteins. Photochem. Photobiol., 21, 463—467 (1975). TALLANDINI, L., SALVATO, B., JORI, G. Photochemical effects associated with the copper absorption bands of the native hemocyanin from Octopus vulgaris. FEBS Lett.,54, 283-285 (1975) ZATTA, P., VESSEY, D.A., ZAKIM, D. The transfer of galactose from UDP-Calactose to endogenous lipid acceptor in liver microsomes. Biochim. Biophys. Acta, 392, 361 (1975). (1976) COLAUTTI, P., MOSCHINI, C., STIEVANO, B.M., TRECNAGHI, C., JORI, C. A study of gamma and neutron interaction with aqueous solutions of L-phenylalanine. J. Radioanal. Chem., 34, 9-17 (1976). GHIRETTI-MAGALDI, A., TAMINO, G., SALVATO, B. The monophyletic origin of hemocyanins on the basis of the amino acid composition. Structural implications. Boll. Zool., 42, 167 (1976). SALVATO, B., JORI, C. A fluorescence study of the copper-protein ceruloplasmin. Bull. Mol. Biol. Med., 1, 97 (1976). TAMBURRO, A.M., SALVATO, B., ZATTA, P. A circular dichroism study of some hemocyanins. Comp. Biochem. Physiol., 55, 347 (1976). ZATTA, P. Heavy metal hydrolisis of poly-isopranoid-phosphate mono and al igosaccarides. Experientia, 32, 693 (1976). ZATTA, P., VESSEY,. D.A. Resolution of endogenous lipid intermediates of glycoproteins synthesis in liver microsomes. Fed. Proc., 35, 948 (1976). ZATTA, P., ZAKIM, D., VESSEY, D.A. Incorporation of N-Acetylglucosamine into lipid linked oligosaccarides. Biochim. Biophys. Res. Comm., 70, 1014 (1976). ZATTA, P., ZAKIM, D., VESSEY, D.A. The lipid intermediates arising during glycoprotein biosynthesis in liver microsomes. Biochirn. Biophys. Acta, 441, 103 (1976). (1977) ALBERGONI, V., FAVERO, N., GHIRETTI, F. The action of D-penicillamine on cytochrome oxidase in vitro. Experientia, 33, 17 (1977). DI STEFANO L., MEZZASALMA V., PIAZZESE S., C.RUSSO G., SALVATO B. The subunit structure of chlorocruorin. F.E.B.S. Letters, 79, 337 (1977). GHIRETTI-MAGALDI, A., GHIRETTI, F., SALVATO, B. The evolution of hemocyanin. In: Biology of Cephalopods. (M.Nixon and J. B.Messenger Eds.) Symp. Zool. Soc. London, 38, 513, Academic Press New York, GHIRETTIMAGALDI, A., TAMINO, G. Evolutionary studies on hemocyanins. In: Structure and Function of Hemocyanin (Bannister, J.V., ed.) Springer Verlag, Berlin, p. 271 (1977). GHIRETTI, F., GHIRETTI-MAGALDI, A. The effect of vitamin C on the intracellular oxygen transport. 31 Intern. Z Vitamin und Ernahrungsf., 16, 41 (1977). GHIRETTI-MAGALDI, A., MILANESI, C., TOGNON, G. Hemopoiesis in Crustacea Decapoda: origin and evolution of hemocytes and cyanocytes of Carcinus maenas. Cell Diff., 6, 167 (1977). FERIGO E., CARDELLINI P., RODINÒ E., SALA M., SALVATO B. Chemical changes in Xenopus laevis haemoglobin during metamorphosis. Acta Embryologiae Experimentalis 2, 137 (1977) JORI, G., SALVATO, B., TALLANDINI, L. Photooxidative and spectral studies of Octopus vulgaris hemocyanin. In: Structure and Function of Hemocyanin (Bannister, J.V., ed.) Springer Verlag, Berlin, pp. 156-163 (1977). SALVATO, B., RICCHELLI, F. The minimal subunit of arthropod hemocyanin. In: Structure and Function of Hemocyanin (Bannister, J.V., ed.) Springer Verlag, Berlin, p. 113 (1977). SALVATO, B., TALLANDINI, L. Oxygen binding of associated and dissociated Octopus vulgaris hemocyanin. In: Structure and Function of Hemocyanin (Bannister, J.V., ed.) Springer Verlag, Berlin, p. 217-230 (1977). SALVATO, B., ZATTA, P. Kinetics of Reaction between hemocyanin and CN and of reconstruction of hemocyanin with K 3Cu(CN)4. In: Structure and Function of Hemocyanin (Bannister, J.V., ed.) Springer Verlag, Berlin, p. 245 (1977). SALVATO, B., ZATTA, P. The binding capacity of hemocyanins of sodium dodecylsulphate. Comp. Biochem. Physiol., 608, 107 (1977). (1978) ALBERGONI, V., FAVERO, N. Effects of Penicillamine on tissue and enzymatic metallo proteins. Comun.Simp.Intern. sul morbo di Wilson, Buenos Aires (1978). ALBERGONI, V., FAVERO, N., ROCCO, G. Copper Metabolism in the Rat: Effects of Copper Loading and Copper Depletion. Bioinorg. Chem., 0, 431 (1978). CASSINI, A., ALBERGONI, V. The oxidation of Sulphydryl compounds by ceruloplasmin. Bull. Mol. Biol. Med., 3, 79 (1978). GHIRETTI-MAGALDI, A., TAMINO, C. L’origine della vita sulla terra. Piccin Ed., Padova (1978). SALVATO B., ZATTA P. The binding capacity of hemocyanins of Sodium Dodecylsulphate. Comp. Biochem. Physiol., 60B, 107 (1978). (1979) FILIPPI, B., GIUSTI, P. , CIMA, L. , BORIN,G. , RICCHELLI , F. , MARCHIORI , F. Synthetic enkephalins. Addicting properties and conforinational studies in solution. Int. J. Peptide Prot. Res., 14, 34-40 (1979). GHIRETTI-MAGALDI, A., SALVATO, B., TALLANDINI, L., BELTRAMIMI, M. The hemocyanin of Aplysia limacina : Chemical and functional characterization. Comp. Biochem. Physio1., 62A, 579 (1979). JORI G., PIZZI G., REDDI E., TOMIO L., SALVATO B., ZORAT P., CALZAVARA F. Time dependence of hematoporphyrin distribution in selected tissues of normal rats and in ascites hepatoma. Tumori, 1979, 65, 43. RICCHELLI, F., SALVATO, B. The binding of 1-8 ANS to the hemocyanin of Octopus vulgaris. Eur. J. Biochem., 94, 199-205 (1979). RUBALTELLI, F.F., JORI, C., ROSSI, E. Modifications of serum albumin during phototherapy of jaundices newborn babies. The Lancet, ii, 2731 (1979). SALVATO, B., GHIRETTI-MAGALDI, A., GHIRETTI, F. 32 Hemocyanin of Octopus vulgaris. The molecular weight of the minimal functional subunit in 3M urea. Biochemistry, 18, 2731 (1979). VESSEY, D.A., ZATTA, P., ZACHIM, D. Properties of dolichol-phosphate: GDP-Mannose Mannosyltransferase in liver microsomes. Medical Biology, 57, 345 (1979). (1980) CARIELLO, L., SALVATO, B., JORI, G. Partial characterization of suberitine, the neurotoxic protein purified from Suberites domuncula. Comp. Biochem. Physiol., 67B, 337-344 (1980). COZZANI, I., JORI, C. Photooxidation of L-glutamate decarboxylase from Escherichia coli, sensitized by proflavine and by the coenzyme pyridoxal phosphate. Biochim. Biophys. Acta, 623, 84—88 (1980). GHIRETTI, F., GHIRETTI-MAGALDI, A. L’evoluzione a livello molecolare. In: Basi Biol. Med. Mod., Vol. I., Ed. Medico Scientifiche, Torino (1980). JORI, G., ROSSI, E., RUBALTELLI, F.F. Phototherapy-induced covalent binding of bilirubin to serum albumin. Pediat. Res., 14, 1363-1366 (1980). RICCHELLI, F., JORI, G., SHOPOVA, N., BOTEVA, P., CENOV, N. Fluorescence properties of native and chemically modified mesentericopeptidase. J. Peptide Protein Res., 17, 330-337 (1980). RICCHELLI, F., SALVATO, B., FILIPPI, B., JORI, C. Conformational changes of Carcinus maenas hemocyanin induced by urea. Arch. Biochem. Biophys., 202, 277-288 (1980). SALVATO, B., BOCCU’, E., GRANDI, C., FONTANA, A., VERONESE, F.M. Enolase from Bacillus stearothermophilus. Physicochemical characterization and Reconstitution of the active enzyme from dissociated subunits. J. Peptide Prot. Res., 15, 139 (1980). SCONFIENZA, C., VAN DE VORST, A., JORI, G. Type I and Type II mechanism in the photooxidation of L-tryptophan and tryptamine sensitized by hematoporphyrin in the presence and in the absence of sodium dodecyl sulphate micelles. Photochem. Photobiol., 31, 351-358 (1980). ZATTA, P., SALVATO, B. Reconstitution of Carcinus maenas hemocyanin in the presence of Triton Xl00. Ciencia Biologica, 5, 111-112 (1980). (1981) CARIELLO, L., SALVATO, B., JORI, G. The role of the cysteinyl and one of the tryptophyl residues in the neurotoxic action of suberitine. Experientia, 37, 801-803 (1981). COLAUTTI, P., MOSCHINI, C., JORI, G., CALZAVARA, F., TOMIO, L., SOTTI, G. La terapia con radiazioni non convenzionali ionizzanti e non ionizzanti. Quaderni Radiologia, 45, Suppl. 1, 1-56 (1981). GHIRETTI-MAGALDI, A., SALVATO, B., TOGNON, C., MAMMI, M., ZANOTTI, C. Structural studies on molluscan hemocyanin. In: Invertebrate oxygen-binding proteins (Lamy J. and Lamy, J., eds.) Marcel Dekker, New York, p. 393 (1981). GHIRETTI-MAGALDI, A. Struttura delle emocianirie dei molluschi. Quaderni di elicicoltura, 10, 11-22 (1981-82). JORI, G., RICCHELLI, F., SALVATO, B., TALLANDINI, L., CANNISTRARO, S. A study of active site topography of Octopus vulgaris hemocyanin by photochemical techniques. In: Invertebrate Oxygen-binding proteins: Structure, Active site and Function (Lamy J. and Lamy, J., eds.) Marcel Dekker, New York, pp. 621—632 (1981). REDDI, E., RICCHELLI, F., JORI, G. Interagtion of human serum albumin with hematoporphyrin and its Zn + and Fe +_denivatives. J. Peptide Protein Res., 18, 402-408 (1981). RICCHELLI, F., JORI, G., SHOPOVA, M., BOTEVA, R. & GENOV, N. Fluorescence properties of native and chemically modified mesentericopeptidase. 33 Int. J. Peptide Protein Res. 17 : 330-337 (1981). RICCHELLI, F., FILIPPI, B., SALVATO, B. Effects of sulphate ions on the denaturation of Carcinus maenas hemocyanin induced by urea. In: Invertebrate Oxygen-Binding Proteins: Structure, Active Site and Function (Lamy,J. and Lamy,J., eds.) Marcel Dekker, New York, pp. 31-39 (1981). SALVATO, B., BELTRAMINI, N., RICCHELLI, F., TALLANDINI, L. The reaction between Carcinus maenas hemocyanin and cyanide. In: Invertebrate Oxygen-binding proteins: Structure, active site and function, (Lamy, J. and Lamy, J., eds.) Marcel Dekker, New York, pp. 633-643 (1981). ZATTA, P. Protein -lipid interactions in Carcinus maenas hernocyanin. Comp. Biochem. Phys., 69b, 731-735 (1981). ZATTA, P., SALVATO, B. Reconstitution of Carcinus maenas hemocyanin in the presence of Non-ionic detergent. J. Inorg. Biochem., 15, 269-273 (1981). ZATTA, P., SALVATO, B. Acetilazione degli amminoacidi-NH2 terminali nelle emocianine. Boll. Soc. It. Biol. Sper., LVII-l9, 1927-1932 (1981). ZATTA, P., SALVATO, B. The polypeptide chains of Carcinus macnas hernocyanin. In: Invertebrate Oxygen binding proteine (Lamy,J. and Lamy,J., eds.) Marcel Dekker, New York, pp. 151-158 (1981). (1982) BERTOLONI, G., DALL’ACQUA, M., VAZZOLER, M., SALVATO, B., JORI, G. Photosensitizing action of hematoporphyrin on some bacterial strains. Med. Biol. Environ., 10, 237-242 (1982). GENOV, N., SHOPOVA, M., BOTEVA, R., JORI, G., RICCHELLI, F. Chemical, photochemical and spectroscopic characterization of an alkaline protease from Bacillus subtilis variant DY. A comparison with other subtilisins. Biochem.J., 207, 193-200 (1982). JORI, C., REDDI, E., RUBALTELLI, F.F. Bronze baby syndrome: evidence for increased serum porphyrin concentration. The Lancet, i, 1072 (1982). REDDI, E., ROSSI, E., JORI, C., SALVATO, B., TOMIO, L., PACINI, P. Photodamage of biological systems upon irradiation with He/Ne laser in the presence of hematoporphyrin. Med. Biol. Environ., 10, 251-258 (1982). RICCHELLI, F. Probing the conformation of hemocyaniris in spectroscopy. Med. Biol. Environnement, 10, 329-331 (1982). RICCHELLI, F., JORI, C., FILIPPI, B., BOTEVA, P., SHOPOVA, M., GENOV, N. Effects of pH and urea on the conformational properties of subtilisin Biochem. J., 207, 201-205 (1982). TOMIO, L, ZORAT, P.L., CALZAVARA, F., COZZANI, I., REDDI, E., SALVATO, B., JORI, C. Cancer phototherapy: biochemical bases and experimental results. Med. Biol. Environ., 10, 301-307 (1982). TOMIO, L., ZORAT, P.L., JOEl, C., REDDI, E., SALVATO, B., CALZAVARA, F. Elimination pathway of hematoporphyrin from normal and tumor-bearing rats. Tumori, 68, 283-286 (1982). (1983) BACCI, M., LINARI, R., RICCIIELLI, F., SALVATO, B. About the origin of a novel fluorescence observed in metallo-proteins. Inorg. Chimica Acta, 79, 276 (1983). GENOV, N., SHOPOVA, M., BOTEVA, R., RICCHELLI, F., JORI, G. Intramolecular distances between tryptophan residues and the active-site serine residues in alkaline bacterial protenases as measured by fluorescence energy-transfer studies. Biochem.J., 215, 413-416 (1983). GHIRETTI, F., GHIRETTI-MAGALDI, A., SALVATO, B. Disturbing results on hemocyanins. In: Structure and Function of Invertebrate Respiratory Proteins (Wood, E., ed.), Harwood Acad. Publ., pp. 397-401 (1983). 34 GHIRETTI, F. The peptidergic nervous transmission. Scienza e Cultura, p. 313 (Dicembre, 1983). RICCHELLI, F., SALVATO, B., GHIRETTI-MAGALD1, A., MEZZASALMA, V., ZAGRA, M., DI STEFANO, L. Preparation of Apochlorocruorin from Spirographis spallanzani. A preliminary conformational characterization. Life Chemistry Reports, Suppl. 1, 203-204 (1983). RICCHELLI, F., TEALDO, E., SALVATO, B. Differential quenching effect of the copper ions in the active site on the hemocyanin fluorescence. Life Chemistry Reports, Suppl. 1, 301-304 (1983). RICCHELLI, F., JORI, C., SHOPOVA, N., BOTEVA, R., QENOV, N. Lanthanide spectral properties as a probe of Ca -binding sites in Mesenterico-peptidase. Inorg. Chimica Acta, 79, 154 (1983). RICCHELLI, F., ROSSI, E., SALVATO, B., JORI, C., BANNISTER, J.V., BANNISTER, W.H. Fluorescence studies on copper/zinc SOD from bovine erythrocytes. In: Oxy Radicals and their scavenger systems (Cohen C. and Greenwald, R.A., eds) Elsevier Science Publ. Co., Vol. 1 , pp. 320-323 (1983). RUBALTELLI, F.F., JORI, C., REDDI, E. Bronze baby syndrome: a new porphyrin-related disorder. Pediat. Res., 17, 327-330 (1983). SALVATO, B., JORI, G., PIAZZESE, A., GHIRETTI, F., BELTRAMINI, N., LERCH, K. Enzymatic activities of type-3 copper pair in Octopus vulgaris hemocyanin. In: Structure and function of Invertebrate respiratory proteins (Wood, E., ed.) Harwood Acad. Publ., London, pp. 313-317. (1983) SALVATO, B., TEALDO, E., BELTRAMINI, M., PEISACH, J. Cobalt hemocyanins. In: Structure and function of Invertebrate respiratory proteins (Wood, E., ed.) Harwood Acad. Publ., London, pp. 291-294 (1983). SALVATO, B., ZATTA, P. The 11 S component of molluscan hemocyanin. In: Structure and function of Invertebrate respiratory proteins (Wood, E., ed.) Harwood Acad. Pubi., London, pp. 139-140 (1983) TALLANDINI, L., CASSINI, A., RICCHELLI, F., FILIPPI, B. Chemical and spectroscopic characterization of a copper-zinc superoxide dismutase from a marine bivalve mollusc ( Mytilus galloprovincialis ). In: Oxy Radicals and their scavenger systems (Cohen G. and Greenwald, R.A., eds.) Elsevier Science Publ. Co., Vol. ]. , pp. 324-327 (1983). TONTO, L., ZORAT, P.L., CORTI, L., CALZAVARA, F., REDDI, E., JOEl, C. Effect of hematoporphyrin arid red light on A1I-l30 solid tumors in rats. Acta Radiologica Oncology, 22, 49-53 (1983). ZATTA P., RICCHELLI F. Metal-protein interaction in Carcinus maenas hemocyanin. Inorg. Chim. Acta. 79: 155-156. (1983) ZATTA, P., GIIIRETTI, F. Non respiratory function of hemocyanin. In: Structure and function of invertebrate respiratory proteins (E. Wood, ed.) Harwood Acad. Publ., London, p. 329 (1983). ZATTA, P., MOSCHINI, C., BUSO, P., COLAUTTI, P., STIEVANO, B.M. Hemocyanin as metal transport protein in Carcinus maenas blood. In: Structure and function of Invertebrate respiratory proteins (E. Wood, ed.) Harwood Acad. Pubi., London, p. 33 (1983). ZATTA, P. Elementi in traccia e sistemi biologici. Ambiente, Risorse, Salute, 12 (Marzo 1983). (1984) BELTRAMINI, M., RICCHELLI, F., PIAZZESI, A., BAREL, A., SALVATO, B. Removal of copper from Octopus vulgaris hemocyanin. Preparation of Half-apo and apo-derivatives. Biochem. J., 221, 911-914 (1984). BELTRAMINI, M., RICCHELLI, F., SALVATO, B. The kinetics of the reaction of Octopus vulgaris hemocyanin with cya~de. Its significance for the structure of the li S subunit of molluscan hemocyanin. Inorg. Chimica Acta, 92, 209-217 (1984). BELTRAMINI, M., RICCHELLI, F., TALLANDINI, L., SALVATO, B. 35 The reaction between cyanide and the hemocyanin of Carcinus rnaenas. A kinetic study. Inorg. Chimica Acta, 92, 219-227 (1984). BERTOLONI, G., DALL’ACQUA, M., VAZZOLER, M., SALVATO, B., JORI, G. Bacterial and yeast cells as models for studying hematoporphiyrin photosensitization. In: Porphyrins in Tumor Phototherapy (Andreoni, A. and Cubeddu, R., eds.) Plenum Press, New York, pp. 177-183 (1984). BERTOLONI, G., SALVATO, B., DALL’ACQUA, M., VAZZOLER, M., JORI, G. Hematoporphyrin-sensitized photoinactivation of Streptococcus faecalis. Photochem. Photobiol., 39, 811-816 (1984). BERTOLONI, G., SALVATO, B., JORI, G. Photosensitization of microbes by hematoporphyrin. Med. Biol. Environ., 12, 435-440 (1984). GHIRETTI, F. Cell Physiology in the “pre-membrane” period. Boll. Soc. It. Biol. Sper., 14, 11 (1984). GHIRETTI, F. Otto Warburg. History and Phylosophy of Life Sciences, 5, 221 (1984). GHIRETTI-MAGALDI, A. L’evoluzione delle proteine. Le Scienze: Gli albori della vita, p. 65 (1984). GHIRETTI-MAGALDI, A., GHIRETTI, F. Metabolismo. Flussi di materia ed energia. In: Trattato di Biologia (Chieffi, G., Baccetti, B. and Nicoletti, B., eds.), Cap. 9, Grasso, Bologna JORI, G., BELTRAMINI, M., REDDI, E., SALVATO, B., PAGNAN, A., ZIRON, L., TOMIO, L., TSANOV, T. Evidence for a major role of plasma lipoproteins as hematoporphyrin carriers in vivo Cancer Letters, 24, 291-297.(1984) MEZZASALMA, V., DI STEFANO, L., PIAZZESE, S., ZAGRA, 1.1., GHIRETTI-MAGALDI, A., CARBONE, R., SALVATO, B. Structural studies on Spirographis Spallanzani chlorocruorin. and In: Structure Function of Invertebrate Respiratory Proteins (Wood, E., ed.) Harwood Acad. PubI. (1984) RICCHELLI, F., GROSSWEINER, L.I. Properties of a new state of hematoporphyrin in dilute aqueous solution. Photochem. Photobiol., 40, 599-606 (1984). RICCHELLI, F., JORI, C., TALLANDINI, L., ZATTA, P., BELTRANINI, N., SALVATO, B. The role of copper and quaternary structure on the conformational properties of Octopus vulgaris hemocyanin. Arch. Biochem. Biophys., 235, 461-469 (1984). ZATTA, P. Sulla partecipazione dell’emocianina all’osmoregolazione del Carcinus maenas. Boll. Zool., 51, 117 (1984) ZATTA, P. Zinc transport in the hemolymph of Carcinus maenas. J. Mar. Biol. Ass. U.K., 64, 801-807 (1984). ZATTA, P. L’ambiente interno. Ambiente, Risorse, Salute, 39 (Ottobre 1984). ZATTA, P., MILANESI, C. Ultrastrutture delle branchie di Carcinus maenas. Boll. Soc. It. Biol. Sper., LX-7, 1385 (1984). ZATTA, P., PICCOLI, A., BUSO, P., PECORARO, M., MOSCHINI, G. Il Selenio: Biologia e tecniche nucleari di rilevamento. Ambiente, Risorse, Salute, 34-36 (Novembre 1984). ZATTA, P., PICCOLI, A., BUSO, P., PECORARO, N., MOSCHINI, C. Selenio e carcinogenesi Ambiente, Risorse, Salute, 37-38 (Novembre 1984). ZATTA, P., SALVATO, B., DI STEFANO, L. Physico-chemical characterization of Squilla mantis hemocyanin. Oebalia, Vol. X, 141 (1984). 36 (1985) BACCI, M., LINARI, R., RICCHELLI, F. & SALVATO, B. A new fluorescence from carboxylic and amino acids. Il Nuovo Cimento, Vol. 6D N.5 : 393-404 (1985). BAREL, A., BELTRAMINI, M., GARBO, G.M., PERIN, A., REDDI, E., ROMANDINI, P., JORI, G. In vitro and in vivo studies on the mechanisms of porphyrin photosensitization of tumor cells. Med. Biol. Environ., 13, 19-23 (1985). BELTRAMINI, M., PIAZZESI, A., ALVIGGI, M., RICCHELLI, F., SALVATO, B. Cobalt substitutions on copper proteins. Med. Biol. Environ., 13, 31-35 (1985). BELTRAMINI, M., RICCHELLI, F., JORI, G., ZIRON, L., PAGNAN, A. Interaction of hematoporphyrin with isolated lipoprotein fractions: a spectroscopic study. In: Photodynamic Therapy of Tumors and Other Diseases (Jon, C. and Perria, A., eds.) Libreria Progetto, Padova, pp. 81-84 (1985) GHIRETTI-MACALDI, A., ZANOTTI, G., TOGNON, C., MEZZASALMA, V. The molecular architecture of the extracellular haemoglobin of Ophelia bicornis. Biochim. Biophys. Acta, 829, 144-149 (1985). JORI, C., PIZZI, G.B., REDDI, E., TOMIO, L., SALVATO, B., ZORAT, P.L Molecular and cellular mechanisms in photomedicine: porphyrins in microheterogeneous environments. In: Primary Photo-processes in Biology and Medicine (Bensasson, R.V., Jon, G., Land, E.J., Truscott, T.G., eds.) Plenum Press, New York, pp. 349—355 (1985). MEZZASALMA, V., DI STEFANO, L., PIAZZESE, S., ZAGRA, M., SALVATO, B., TOGNON, G., GHIRETTI-MAGALDI, A. Physicochemical and structural properties of the extracellular haemoglobin of Ophelia bicornis. Biochim. Biophys. Acta, 829, 135-143 (1985) RICCHELLI, F., ZATTA, P. Sectroscopic properties of 50.000 D subunit of Octopus vulgaris hemocyan in. Medicine Biologie Environ., 13, 105—108 (1985). RICCHELLI, F. & JORI, G. Spectroscopic studies on the intraliposomal distribution of porphyrins. In : Photodynamic therapy of tumors and other diseases ( Jori,G. & Perria,C.A., eds. ), Libreria Progetto, Padova, pp. 85-87 (1985). RICCHELLI, F., ZATTA, P. Sectroscopic properties of 50.000 D subunit of Octopus vulgaris hemocyan in. Medicine Biologie Environ., 13, 105-108 (1985) ZATTA, P., BUSO, P., MOSCHINI, C. Selenium distribution in the tissues of Carcinus maenas. Comp. Biochem. Physiol., 8lC, 469-470 (1985). ZATTA, P., PICCOLI, L. Il Tellurio: tossicità e mielinopatie. Ambiente Risorse Salute, 40, 32-33 (1985) ZATTA, P., Interaction between Zn2+, Co2+, Mn2+ with hemocyanin from Carcinus maenas. Cahiers Biol. Mar. XXVI: 241-249. (1985) (1986) BANCI , L. , BERTINI , I . , LUCHINAT, C. , MONANNI , R. , SCOZZAFAVA,A. , SALVATO, B. A spectroscopic investigation of Co2-Zn2 and Co2-Co2 superoxide dismutase. Gazz. Chim. Ital., 116, 51-54 (1986). BAREL, A. , BELTRAMINI , M. , I G. , MENEGAZZO, E. , PERIN, A. , REDDI , E., RICCHELLI , F . , BELTRAMINI, M., PIAZZESI, A., ALVIGGI, M., RICCHELLI, F., SALVATO, B. Effects of solvent composition on hemocyanin conformation. In: Invertebrate Oxygen Carriers (Linzen, B., ed.) Springer-Verlag, Berlin, pp. 429-433 (1986). BELTRAMINI, M., PIAZZESI, A., ALVIGGI, M., RICCHELLI, F., SALVATO, B. Half-apo derivatives of hemocyanins. In: Invertebrate Oxygen Carriers (Linzen, B., ed.) Springer-Verlag, Berlin, pp. 437-440 (1986). GHIRETTI, F. 37 Homage to Rene’ Lontie. In: Invertebrate oxygen carriers (B. Linzen, ed.), Springer Verlag, Berlin, p. 3 (1986). GHIRETTI, F. Comparative Physiology and Biochemistry at the Zoological Station of Naples. Biol. Bull., 168, 122 (1986). GHIRETTI, F. Francois Magendie, pioniere della Scienza della Nutrizione. Rass. Med. Sper., 33, 249 (1986). GHIRETTI, F. La Fisiologia fra Biofisica e Biochimica. Rass. Med. Sper., 32, 249 (1986). GHIRETTI, F. La Fisiologia fra Biofisica e Biochimica. Rass. Med. Sper., 32, 249 (1986). GHIRETTI-MAGALDI, A., GHIRETTI, F., TOGNON, G., ZANOTTI, G. The structure of the extracellular hemoglobin of Annelids. In: Invertebrate oxygen carriers (B. Linzen, ed.), Springer Verlag, Berlin, pp. 45—52 (1986). GHIRETTI-MAGALDI, A., TOGNON, G. Ophelia bicornis erythrocruorin. Some peculiar features. In: Invertebrate oxygen carriers (B. Linzen, ed.), Springer Verlag, Berlin, pp. 53-55 (1986) PAGOTTO, C., ZATTA, P. La pesca del Pecten jacobaeus nell’Alto Adriatico. Ambiente, Risorse, Salute, Gennaio, 33-35 (1986). RICCHELL1, F., FILIPPI, B., GOBBO, S., SIMONI E., TALLANDINI, L., ZATTA, P. Functional and structural properties of the 50.000 D subunit of Octopus vulgaris hemocyanin. In: Invertebrates oxygen carriers (Linzen, B., ed.) Springer Verlag, Berlin (1986). RICCHELLI, F., BELTRAMINI, M., FLAMIGNI, L., SALVATO, B. Fluorescence quenching mechanism in hemocyanin. Med. Biol. Environ., 14, 169-172 (1986). RICCHELLI, F., FILIPPI, B., GOBBO, S., SIMONI E., TALLANDINI, L., ZATTA, P. Functional and structural properties of the 50.000 D subunit of Octopus vulgaris hemocyanin. In: Invertebrates oxygen carriers (Linzen, B., ed.) Springer Verlag, Berlin, pp. 235-239 (1986) RICCHELLI, F., JORI, G. Distribution of Porphyrins in the various compartments of unilamellar liposomes of dipalmitoylphosphatidylcholine as probed by fluorescence spectroscopy. Photochem. Photobiol., 44, 151-157 (1986). ROMANDINI , P . , VALDUGA , G. Photosensitizing and phototherapeutic properties of porphyrins and phthalocyanines. Med. Biol. Environ., 14, 35-40 (1986). SALVATO, B., BELTRAMINI, M., PIAZZESI, A., ALVIGGI, M., RICCHELLI, F. Spectroscopic characterization of a Co(II) derivative of Carcinus maenas hemocyanin. In: Invertebrate Oxygen Carriers (Linzen, B., ed.) Springer-Verlag, pp. 441-444 (1986). SALVATO, B., BELTRAMINI, M., PIAZZESI, A., ALVIGGI, M., RICCHELLI; F., MAGLIOZZO, R.S., PEISACH, J. Preparation, Spectroscopic Characterization and Anion Binding Studies of a Mononuclear Co (II) Derivative of Carcinus maenas Hemocyanin. Inorg. Chim. Acta, 125, 55-62 (1986) SESTI, A. G., BOMBI, B., CORAIN, B., GIORDANO, R., STELLA, R., ZATTA, P. Alluminio ambientale e suoi possibli rapporti con alcune encefalopatie umane. Biologi italiani, XVI, 22-26 (1986). ZATTA, P. Interaction between Zn , Co , Mn , with hemocyanin from Carcinus maenas. Cahiers de Biol. Mar., XXVI, 241-249 (1986). (1987) BELTRAMINI, M., FIREY,P.A., RICCHELLI,F., RODGERS,M.A.J., JORI ,G. 38 Steady-state and time-resolved spectroscopic studies on the hematoporphyrin-lipoprotein complex. Biochemistry, 26, 6852-6858 (1987). RICCHELLI , F., BELTRAMINI , M., FLAMIGNI , L., SALVATO, B. Emission quenching mechanisms in Octopus vulgaris hemocyanin: steady-state and time-resolved fluorescence studies. Biochemistry, 26, 6933-6938, (1987). ROMANDINI , P., BAREL, A., BELTRAMINI, M., SALVATO, B. The kinetics of reconstitution of Carcinus maenas apohemocyanin. J. Inorg. Biochem., 30, 253-259 (1987). SANSONE, G., COTUGNO, M., COSMA, I, ZATTA, P. The effect of alanine on the concentration of taurine and other free amino acids during the hypoosmotic stress in Mytilus galloprovincialis. J. Mar. Biol. Ass. U.K., 67, 111-117 (1987). SPEZIALI M., ORVINI E., RIZZIO E., GIORDANO R., PERAZZOLO M., ZAPPA P. ZATTA, P. Atrazina. Appunti di tossicologia e considerazioni generali. Ambiente Risorse Salute, Maggio (1987). ZATTA, P. Dopamine, noradrenaline and serotonin duringh hypo-osmotic stress of Carcinus maenas. Marine Biol., 96, 479-481 (1987). ZATTA, P., CERVELLIN, D. Hypo-osmotic stress in the bivalve mollusc Callista chione (Lam.). Monitore Zool. Ital., 21 (4), 287-292 (1987). ZATTA, P., GIORDANO, R., CORAIN, B., FAVARATO, M., BOMBI, G.G. A neutrophilic compound of aluminum (III) as a cause of myocardial infarct in the rabbit. Toxicol. Letters, 39, 185-188 (1987). ZATTA, P. Calcium-taurine interaction in the heart of the crab Carcinus maenas. Gallium determination in different human brain sites. In: Current Trends in Trace Element Research. Smith-Gordon and Co Ltd, London, UK, pp 64-65. (1987) ZATTA, P. The relationship between plasma proteins and intracellular free amino acids during the hypo-osmotic regulation of Carcinus maenas. J.Exp. Zool., 242, 131-136 (1987). ZATTA, P. Kinetics of rection between hemocyanin and CN J.Inorg. Biochem., 30, 247-250 (1987). Canadian J. Fish. Aquatic Sc. 44(10): 1765-1768. (1987) ZATTA, P. , MILANESI C. Ultrastruttura delle branchie di 4 specie di crostacei decapodi: Carcinus maenas, Cancer pagurus, Homarus americanus, Austropotamobius pallipes italicus. Nova Thalassia 8 (Suppl. 3) 589- 592. (1987) (1988) BELTRAMINI, M., SANTAMARIA, M. SALVATO, B. Effects of anions Ca , Mg , and aliphatic alcohols on the reaction of hemocyanin with cyanide. Arch. Biochem. Biophys., 262, 149-158 (1988). CORAIN B., BOMBI G.G., ZATTA, P. Differential effects on covalent compounds in aluminum toxicology. Neurobiol. Aging. 9: 413-414. (1988) CORAIN B., ZATTA, P., BOMBI G.G., GIORDANO R. Cardiotoxicity of the lipophilic compound aluminum acetylacetonate. Biomed. Environm. Sciences 1: 283-287. (1988) GIACOMETTI, G.M., DI MURO, P., SALVATO, B., BELTRAMINI, M. The reaction of Octopus vulgaris hemocyanin with exogeneous ligands. Proposal of an allosteric model for the binding of cyanide and thiourea to the 11S component. Arch. Biochem. Biophys., 266, 539-547 (1988). RICCHELLI , F., BELTRAMINI, M., BOTEVA, R., SALVATO, B. Luminescence processes associated with the binding of carbon monoxide with hemocyanin. Med. Biol. Environ., 16, 51-56 (1988). RICCHELLI , F., BIOLO, R., REDDI , E., TOGNON, G. 39 Liposomes as carriers of hydrophobic photosensitizers in vivo: increased selectivity of tumour targetting. In: New Directions in Photodynamic Therapy. (Neckers,.C., ed.) SPIE Publisher, Cambridge, Massachusetts, pp. 101-106 (1988). RICCHELLI , F., OLSEN,K., LINDQVIST, L.Influence of the gel-liquid transition on hematoporphyrin triplet derivation in liposomes. J. Photochem. Photobiol., 2, 475-481 (1988). RICCHELLI, F., STEVANIN, D. JORI, G. Porphyrin-liposome interactions: influence of the chimico-physical properties of the phospholipid bilayer. Photochem. Photobiol., 48, 1-5 (1988). ZATTA, P., FAVARATO, M., BOMBI , G. G. L’uso di pesticidi in Italia: riflessioni, analisi, proposte. Ambiente Risorse Salute, febbraio, 11-13 (1988). ZATTA, P., GIORDANO, R., CORAIN, B., BOMBI , G. G. Alzheimer dementia and aluminum hypothesis. Med. Hypoth., 26, 139-142 (1988). (1989) BASSI,R., GHIRETTI-MAGALDI, A., TOGNON, G., GIACOMETTI, G.M. AND MILLER, K.R. Two dimensional crystals of photosystem II reaction center complex from higher plants. Eur. J. of Cell Biol. 50, p. 84-93, (1989). BELTRAMINI, M., GIACOMETTI, G.M., SALVATO, B., GIACOMETTI, G., MUNGER, K., LERCH, K. Luminescence emission from Neurospora copper metallothionein: time resolved studies. Biochem. J. 260, 189-193 (1989). CEJKA, Z., GHIRETTI-MAGALDI ,A., TOGNON, G., ZANOTTI ,G. AND BAUMEISTER W. The molecular architecture of extracellular Haemoglobin of Eophila Tellinii. J. Ultrastr. Mol. Str. Res. 102, p. 71-81, (1989). GAVA, C., PERAZZOLO ,M., ZENTILIN, L., LEVIS ,A.G., CORAIN ,B., BOMBI ,G.G., PALUMBO, M. AND ZATTA, P. Genotoxic potentiality and DNA-binding properties of acetylacetone, maltol, and their Aluminum(III) and Chromium (III) neutral complexes. Toxicol.Environm.Chem. 22: 149-157.(1989) PAGANELLI, M., ROMANDINI, P. BERTOLONI, G., BELTRAMINI, M., TALLANDINI, L., SALVATO, B. Induction of superoxide-dismutase by methanol and strucutral modifications in Candida albicans. Yeast, Spec. Iss. 0431-05, S431-S435 (1989). RICCHELLI, F., BELTRAMINI, M., SALVATO, B. 1-anilino-8-naphtalene sulphonate as fluorescent probe of metal active sites of laccase. Med. Biol. Environ. 17, 855-859 (1989). SALVATO, B., GIACOMETTI, G.M., BELTRAMINI, M., ZILIO, F., GIACOMETTI, G., MAGLIOZZO, R.S., PEISACH, J. The oxidation of Octopus vulgaris hemocyanin by nitrogen oxides. Biochemistry 28, 680-684 (1989). SALVATO, B., BELTRAMINI, M., RICCHELLI, F., TALLANDINI, L. Cobalt substitution on bovine erythrocyte superoxide-dismutase: evidence for a novel cobaltsuperoxide dismutase derivative. Biochim. Biophys. Acta 998, 14-20 (1989). SPEZIALI, M., RIZZIO ,E., ORVINI ,E., RIZZO, E., GIORDANO, R., ZATTA ,P., FAVARATO, M. AND PERAZZOLO ,M. Gallium distribution in different human brain areas. Biol. Trace Elem. Res. 22: 9-15. (1989) VALLE, G.C., BOMBI, G.G., CORAIN, B., FAVARATO, M. AND ZATTA, P. Crystal and molecular structures of Diaqua-(nitrilotriacetate) aluminum(III) and Di-m-hydroxo-bis (nitrilotriacetate) dialuminate (III) dianion. J.Chem.Soc.Dalton Trans. 1513-1515. (1989) ZATTA, P., PERAZZOLO, M. AND CORAIN, B. Tris acetylacetonate aluminum(III) induces osmotic fragility and acanthocytes formation in suspended erythrocytes. Toxicol. Letters 45: 15-21.(1989) 40 ZATTA, P., PERAZZOLO ,M., BOMBI, G.G., CORAIN, B. AND NICOLINI, M. The role of speciation in the effects of aluminum(III) on the stability of cell membranes and on the activity of selected enzymes. In: "Alzheimer's Disease and Related Disorders". Progress in Clinical and Biological Research", vol. 317 . Iqbal K., Wisniewski H., Winblad B (Eds), Alan Liss Inc., New York, pp. 1087-1094.(1989) (1990) BELTRAMINI, M., SANTAMARIA, M., SALVATO, B., LERCH, K. Folding of the polypeptide chain (s), conformational flexibility and reactivity of the metal active site of hemocyanin and tyrosinase. Biol. Metals 3, 93-97 (1990). BELTRAMINI, M., SALVATO, B., SANTAMARIA, M., LERCH, K. The reaction of CN - with the binuclear copper site of Neurospora tyrosinase: its relevance for a comparison between tyrosinase and hemocyanin active sites. Biochim. Biophys. Acta 1040, 365-372 (1990). BOMBI, G.G., CORAIN ,B., FAVARATO, M., GIORDANO, R., NICOLINI, M., PERAZZOLO ,M., TAPPARO, A. AND ZATTA ,P. Experimental aluminum pathology in rabbit: effect of hydrophilic and lipophilic compounds. Environm. Health Perspect. 89: 217-222.(1990). BOMBI, G.G., CORAIN, B., TAPPARO, A., ZATTA, P., NICOLINI M., PERAZZOLO, M. AND FAVARATO, M. Alzheimer's disease and aluminum toxicology. Environm. Health Perspect. 89: 233-235 (1990). BOTEVA, R., SEVEROV, S., GENOV., N., FILIPPI, B., BELTRAMINI, M., RICCHELLI, F., PALLHUBER, M.A., TALLANDINI, L, TOGNON, G., SALVATO, B. General characterization of the hemocyanin from the gastropod Rapana thomasiana. In "Invertebrate Dioxygen Carriers" (Preaux, G., Lontie, R., eds.), Leuven Univ. Press, Leuven, pp. 93-98 (1990). BOTEVA, R., SEVEROV, S., BELTRAMINI, M., BUBACCO, L., GIACOMETTI, G.M., SALVATO, B. Ligand binding on the hemocyanins from Octopus vulgaris (Octopoda) and Rapana thomasiana (Gastropoda) In "Invertebrate Dioxygen Carriers" (Preaux, G., Lontie, R., eds.), Leuven Univ. Press, Leuven, pp. 417-422 (1990). BUBACCO, L., BELTRAMINI, M., SALVATO, B., MAGLIOZZO, R.S. Cobalt substituted derivatives of Carcinus hemocyanin. Biol. Metals 3, 90-92 (1990). BUBACCO, L., BELTRAMINI, M., SALVATO, B., PEISACH, J., MAGLIOZZO, R.S. Co(II)-substitution studies of Carcinus maenas hemocyanin. In "Invertebrate Dioxygen Carriers" (Preaux, G., Lontie, R., eds.), Leuven Univ. Press, Leuven, pp. 381-385 (1990). FAVARATO, M. AND ZATTA , P. Chemico-clinical characterization of rabbit serum. J. Appl. Rabbit Res. 13: 14-15. (1990) PERAZZOLO ,M., FONTANA, L., FAVARATO, M., CORAIN, B., BOMBI, G.G., TAPPARO, A., NICOLINI ,M., CORVAJA, C., ZATTA, P. Effects of aluminum(III) on plasmatic membranes . In:" Metal ions in Biology and Medicine". Collery P., Poiries L.A., Mainfait M. and Etienne J.C.(Eds) J.Libbey Eurotext, Paris pp 326-328.(1990) F. RICCHELLI, G. JORI, G. MORENO, F. VINZENS, C. SALET. Factors influencing the distribution pattern of porphyrins in cell membranes. J. Photochem. Photobiol., B:Biol. 6:69-77 (1990) RICCHELLI, F. An investigation of the electronic environment of tryptophan in proteins : reliability of the fluorescencen quenching experiments. J. Photochem. Photobiol., B: Biol. 7 : 93-100 (1990). SANTAMARIA, M., BELTRAMINI, M., SALVATO, B. Catalytic properties of the binuclear copper active site of hemocyanin. In "Invertebrate Dioxygen Carriers" (Preaux, G., Lontie, R., eds.), Leuven Univ. Press, Leuven, pp. 437-440 (1990). (1991) BAZZATO G., MORACCHIELLO P., ZATTA P., FAVARATO M. Alterazioni morfologiche eritrocitarie "microcytic-like" in corso di trattamento dialitico. 41 Talassemia e Rene, 43-46. (1991) BOTEVA, R., SEVEROV, S., GENOV, N., BELTRAMINI, M., FILIPPI, B., RICHELLI, F., TALLANDINI, L., PALLHUBER, M., TOGNON, G., SALVATO, B. Biochemical and functional characterization of Rapana thomasiana hemocyanin. Comp. Biochem. Physiol. 100B, 493-501 (1991). CEJCA, Z., SANTINI, C., TOGNON, G. AND MAGALDI, A. The molecolar architecture of the extracellular Haemoglobin of Ophelia Bicornis. Analisis of two dimensional crystalline arrays. J. Struct. Biol. 107, p. 257-259 , (1991). FONTANA L., PERAZZOLO M., STELLA M. P., TAPPARO A., CORAIN B., FAVARATO M., ZATTA P. A long-term toxicological investigation on the effect of tris(maltolate)aluminum (III) in rabbits. Biol. Trace Elem. Research. 31:183-191. (1991) LA MONACA, A., BARTERI, M., BORGHI, E., CONGIU-CASTELLANO, A., CAPPUCCIO, G., BELTRAMINI, M., SALVATO, B., SHAH, J.S. Application of the improved area detector for continous small angle X-ray scattering using synchroton radiation. Nucl. Instr. Meth. Phys. Res. A 310, 403-410 (1991). LA MONACA, A., BARTERI, M., BORGHI, E., BELTRAMINI, M., SALVATO, B., CONGIUCASTELLANO, A., SHAH, J.S. Advanced small-angle X-ray synchroton radiation scattering study of hemocyanin oligomers using a high-performance two-dimensional detector. Chem. Phys. Lett. 184, 1-5 (1991). RICCHELLI, F., JORI, G., GOBBO, S., TRONCHIN, M. Liposomes as models to study the distribution of porphyrins in cell membranes. Biochim. Biophys. Acta 1065:42-48 (1991) ZATTA P., NYAME K., CORMIER M.J., SMITH D.F., MATTOX S., PRIETO P.A., CUMMINGS R.D. A solid-phase assay for -1,4 galactosyltransferase in human serum using recombinant aequorin. Anal. Biochem 194: 185-191. (1991) (1992) BELTRAMINI, M., BUBACCO, L., SALVATO, B., CASELLA, L., GULLOTTI, M., GAROFANI, S. The aromatic circular dichroism spectrum as a probe for conformational changes in the active site environment of hemocyanins. Biochim. Biophys. Acta 1120, 24-32 (1992). BOTEVA, R., RICCHELLI, F., SARTOR, G. & DECKER, H. Fluorescence properties of hemocyanin from tarantula ( Eurypelma californicum ). Med. Biol. Environ. Vol. 20 N.1 : 49-53 (1992). BOTEVA, R., RICCHELLI, F., SARTOR, G. & DECKER, H. Fluorescent probes to study conformational changes of hemocyanin from tarantula Eurypelma californicum Med. Biol. Environ. Vol. 20 N.1 : 57-62 (1992) BUBACCO, L., MAGLIOZZO, R.S., BELTRAMINI, M., SALVATO, B., PEISACH, J. Preparation and spectroscopic characterization of a coupled binuclear center in Co(II) substituted hemocyanin. Biochemistry 31, 9294-9303 (1992). FAVARATO M., ZATTA P., PERAZZOLO M., FONTANA L., NICOLINI M. 14 Aluminum(III) Influences the permeability of the Blood-Brain Barrier to C-sucrose in rats. Brain Research 569: 330-335. (1992) MEZZASALMA ,V., TOGNON ,G., GHIRETTI-MAGALDI, A. The Hemopoietic tissue of Spirographis Spallanzanii An ultrastructural study. Anim. Biol. , 1, p.85-96, (1992). RICCHELLI, F., BELTRAMINI, M., BUBACCO, L., SALVATO, B., FILIPPI, B. Circular dichroism and fluorescence studies to probe the conformational properties of Rhus vernicifera laccase. Inorg. Chim. Acta 193 : 237-243 (1992). RICCHELLI, F., GOBBO, S., TRONCHIN, M., JORI, G., SALET, C., VINZENS, F. , MORENO, G. Photoinactivation of mitochondria : effect of dye-hydrophobicity and dye-carrier. Med. Biol. Environ. Vol. 20 N.1 : 41-46 (1992). 42 ROMANDINI, P., TALLANDINI, L., BELTRAMINI, M., SALVATO, B., MANZANO, M., DE BERTOLDI, M., ROCCO, G.P. Effects of copper and cadmium on growth, superoxide dismutase and catalase activities in different yeast strains. Comp. Biochem. Physiol. 103C, 255-262 (1992). SHOPOVA, M., MANTAREVA,V., KRASTEV, K., HARDJIOLOV, D., MILEV, A., SPIROV, K., JORI, G., RICCHELLI, F. Comparative pharmacokinetic and photodynamic studies with Zn(II)-phthalocyanine in hamsters bearing an induced or transplanted rhabdomyosarcoma. J. Photochem. Photobiol., B:Biol. 16:83-89 (1992) ZATTA P., PERAZZOLO M., FACCI L., SKAPER S.D. CORAIN B., FAVARATO M., Effects of aluminum speciation on murine neuroblastoma cells. Molec. Chem. Neuropathol. 16:11-22. (1992) ZATTA P., GOBBO S., ROCCO P., PERAZZOLO M., FAVARATO M. Evaluation of heavy metal pollution in the Venetian lagoon by using he Mytilus galloprovincialis as biological indicator. The Sci. Total Environm. 119, 29-41. (1992) (1993) BELTRAMINI, M., BORGHI, E., DI MURO, P., GHIRETTI-MAGALDI, A., LA MONACA, A., SALVATO, B., SANTINI, C., TOGNON, G. SAXS on invertebrate dioxygen carriers. Journ. de Physique IV C8 3, 249-252 (1993). BOTEVA,R., RICCHELLI,F., SARTOR,G. & DECKER,H. Fluorescence properties of hemocyanin from tarantula (Eurypelma californicum) : a comparison between the whole molecule and isolated subunits. J. Photochem. Photobiol., B:Biol. 17 : 145-153 (1993). BOTEVA, R., RICCHELLI, F., SARTOR, G., DECKER, H. Labeling of tarantula hemocyanin (Eurypelma californicum) with dansyl-type fluorescent tags : identification of the dye-binding site by fluorescence spectroscopy. J. Photochem. Photobiol., B:Biol. 17 : 155-161 (1993). BUBACCO, L., ROCCO, G.P., SALVATO, B., BELTRAMINI, M. The binding of Cd(II) to the hemocyanin of the mediterranean crab Carcinus maenas. Arch. Biochem. Biophys. 302, 78-84 (1993). DELLA LONGA, S., BIANCONI, A., PALLADINO, L., SIMONELLI, B., CASTELLANOCONGIU, A., BORGHI, E., BARTERI, M., BELTRAMINI, M., ROCCO, G.P., SALVATO, B., BUBACCO, L., MAGLIOZZO, R.S., PEISACH, J. A XANES study of metal coordination in Co(II)-substituted Carcinus maenas hemocyanin. Biophys. J. 65, 2680-2691 (1993). FAVARATO M., ZATTA P.F. Differential Aluminum lactate toxicity in rabbits using either acqueous solution or liposomal suspensions. Toxicol. Lett. 66: 133-146. (1993) IDAKIEVA K., SEVEROV S., SVENDSEN I., GENOV N., STOEVA S., BELTRAMINI M., TOGNON G., DI MURO P. SALVATO B. Structural properties of Rapana thomasiana grosse hemocyanin: isolation, characterization and Nterminal aminoacid sequence of two different dissociation products. Comp. Biochem. Physiol. 106B, 53-59 (1993). LA MONACA A., BORGHI E., BARTERI, M., BELTRAMINI M., SALVATO B., CONGIUCASTELLANO A., SHAH, J.S. Fractal evidence in metallo-protein aggregates by SAXS measurements. Physica Medica 9, Sup.1, 47-51 (1993). MANZANO, M., ROMANDINI, P., DE BERTOLDI, M., BELTRAMINI, M., SALVATO, B., COZZANI, I. Interaction among heavy metals and methanol affecting superoxide dismutase activity in Saccaromyces cerevisiae. Comp. Biochem. Physiol. 105C, 175-178 (1993). MIRZABEKOV T., BALLARIN C., NICOLINI M., ZATTA P., SORGATO M.C. Reconstitution of the native mitochondrial outer membrane in planar bilayer-comparison with the outermembrane in a patch pipette and effect of aluminum compounds. J. Membr. Biol. 133: 129-143. (1993) 43 PELLERITO L., PELLERITO A., MAGGIO F., BELTRAMINI M., SALVATO B., RICCHELLI F. Organometallic complexes with biological molecules, I. Diorganotin(IV)chloro protoporphiryn IX complexes: solid state and solution-phase characterization. Appl. Organom. Chem. 7, 79-84 (1993). RICCHELLI F., GOBBO S., JORI, G., MORENO G., VINZENS F., SALET, C. Photosensitization of mitochondria by liposome-bound porphyrins. Photochem. Photobiol. 58:53-58 (1993) VITRANO E., CUPANE A., LEONE, M., MILITELLO, V., CORDONE L., SALVATO B., BELTRAMINI, M., BUBACCO, L., ROCCO, G.P. Low temperature optical spectroscopy of cobalt-substituted hemocyanin from Carcinus maenas. Eur. J. Biophys. 22, 157-167 (1993). ZATTA P., CERVELLIN D., MATTIELLO G., GEROTTO M., LAZZARI F., GASPARONI G., GOMIRATO L., MAZZOLINI G., SCARPA G., ZANOBONI V., PILONI M.G., FAVARATO M. Plasma multielemental analysis in Alzheimer's disease and multinfarctual dementia. Trace Elem.Med. 10: 85-89. (1993) ZATTA P., BORDIN C., FAVARATO M. The inhibition of trypsin and a-chymotrypsin proteolityc activity by aluminum (III) Arch.Biochem. Biophys. 303: 407-411. (1993) ZATTA P., FAVARATO M., NICOLINI M. Deposition of aluminium in brain tissues of rats exposed to inhalation of aluminium acetylacetonate. NeuroReport 4:1110-1112. (1993) (1994) BELTRAMINI, M., DI MURO, P., ROCCO, G.P., SALVATO, B. Luminescence properties of the dinuclear copper complex in the active site of hemocyanins. Arch. Biochem. Biophys. 313, 318-327 (1994). BRAGADIN M., DELL’ANTONE P., ZATTA P. + Inhibitory effect of aluminum on H -ATPase in isolated lysosomes.In: Aluminium in Chemistry, Biology and Medicine. vol. 2. Life Chemistry Reports (Special issue). Harwood Acad. Publ. GmbH, London pp165-169. (1994) CORAIN B., SHEIK-OSMAN A.K., BERTANI R., TAPPARO A., ZATTA P. The aqueous solution state of a-hydroxycarboxylate complexes of aluminium (III): an IR and NMR approach. In: Aluminium in Chemistry, Biology and Medicine. vol. 2. Life Chemistry Reports (Special issue). Harwood Acad. Publ., GmbH, London, UK, pp.103-109. (1994) DELLINGER, M., RICCHELLI, F., MORENO G. & SALET, C. Hematoporphyrin Derivative ( Photofrin ) Photodynamic Action on Ca2 Transport in Monkey Kidney Cells (CV - 1). Photochem. Photobiol. 60 : 368 - 372 ( 1994 ). FAVARATO M., ZATTA P. Histofluorimetric aluminum determination in Alzheimer's disease brain. In: Aluminium in Chemistry, Biology and Medicine. vol. 2. Life Chemistry Reports (Special issue). Harwood Acad. Publ.,GmbH, London pp.71-78. (1994) MAZZINI, A., BELTRAMINI, M., FAVILLA, R., CAVATORTA, P., DI MURO, P., SALVATO, B. An oxygenation-sensitive dye binding to Carcinus maenas hemocyanin. Biophys. Chem. 52, 145-156 (1994). RICCHELLI, F., NIKOLOV, P., GOBBO, S., JORI, G., MORENO, G., SALET, C.A. Interaction of phthalocyanines with lipid membranes: a spectroscopic and functional study on isolated rat liver mitochondria. Biochim. Biophys. Acta 1196:165-171 (1994) ROMANDINI, P., BONOTTO, C., BERTOLONI, G., BELTRAMINI, M., SALVATO, B. Superoxide dismutase, catalase and cell dimorphism in Candida albicans cells exposed to methanol and different temperatures. Comp. Biochem. Physiol.108C, 53-57(1994). SANTINI, C., TIDU, V., TOGNON, G., GHIRETTI–MAGALDI, A. AND BASSI, R. Three dimensional structure of the higher-plant photosistem II reaction centre and evidence for its dimeric organization in vivo. Eur. Jour. Biochem. 221, p. 307-315, (1994). SARAIS, I., MANZANO, M., DE BERTOLDI, M., ROMANDINI, P., BELTRAMINI, M., SALVATO, B. 44 Adaptation of a Saccaromyces cerevisiae strain to high copper concentrations. Biometals 7 , 221-226 (1994). ZAMBENEDETTI P., TISATO F., ZATTA P. CORAIN B. The reactivity of aluminium(III) with membrane phospholipids. A NMR approach. Biometals 7: 244-252. (1994) ZATTA P., ZAMBENEDETTI P., CERVELLIN D. Neuroblastoma cells: an aluminum(III) cytotoxicological model. In: Aluminium in Chemistry, Biology and Medicine. vol. 2. Life Chemistry Reports (Special issue). Harwood Acad. Publ.,GmbH, London pp.111-123. (1994) ZATTA P., ZAMBENEDETTI P., MASIERO S. Effects of aluminum lactate on murine neuroblastoma. NeuroToxicology 15: 789-798. (1994) ZATTA P., ZAMBENEDETTI P., BRUNA V., FILIPPI B. Activation of acetylcholinesterase by Al(III): relevance of the metal speciation. NeuroReport 5: 1777-1780. (1994) ZATTA P., CERVELLIN D., FAVARATO M., GEROTTO M., MATTIELLO G. Microelemental concentration in the ontogenesis of rat brain. Trace Elem. Med. 11:143-147. (1994) (1995) BELTRAMINI, M., BUBACCO, L., CASELLA, L., ALZUET, G., GULLOTTI, M., SALVATO, B. The oxidation of hemocyanin. Kinetics, reaction mechanism, and characterization of Met-hemocyanin product. Eur. J. Biochem. 232, 98-105 (1995). BUBACCO, L., MAGLIOZZO, R.S., WIRT, M.D., BELTRAMINI, M., SALVATO, B., PEISACH, J. Structural characterization of mononuclear Cu(II) in the active site of Carcinus maenas hemocyanin. Biochemistry 34, 1524-1533 (1995). DELL’ANTONE P., BRAGADIN M., ZATTA P. The accumulation of tacrine in the acidic compartments of the cell. Biochem. Biophys. Acta 1279: 137-141. (1995) MAGLIOZZO, R.S., BUBACCO, L., McCRAKEN, J., JIANG, F., BELTRAMINI, M., SALVATO, B., PEISACH, J. Cu(II) coordination in arthropod and mollusc green half-met hemocyanins analyzed by electron spin echo envelope modulation spectroscopy. Biochemistry 34, 1513-1523 (1995). RICCHELLI, F., GOBBO, S., JORI, G., MORENO, G., SALET, C. Temperature-induced changes in fluorescence properties as a probe of porphyrin microenvironment in lipid membranes. 1. The partition of hematoporphyrin and protoporphyrin in liposomes. Eur. J. Biochem. 233:159-164 (1995) RICCHELLI, F., GOBBO, S., JORI, G., SALET, C., MORENO, G. Temperature-induced changes in fluorescence properties as a probe of porphyrin microenvironment in lipid membranes. 2. The partition of hematoporphyrin and protoporphyrin in mitochondria. Eur. J. Biochem. 233:165-170 (1995) RICCHELLI, F. GOBBO, S. Porphyrins as fluorescent probes for monitoring phase transitions of lipid domains in biological membranes. factors influencing the microenvironment of hematoporphyrin and protoporphyrin in liposomes. J. Photochem. Photobiol. B : Biol.. 29 : 65 - 70 (1995). RICCHELLI, F. Photophysical properties of porphyrins in biological membranes . J. Photochem. Photobiol. B : Biol. 29 : 109 - 118 (1995). SHOPOVA, M., STOICHKOV, N., MILEV, A., PEEV, M., GEORGIEV, K., GIZBREHT, A., JORI, G., RICCHELLI, F. Photodynamic therapy of experimental tumours with Zn(II)-phthalocyanine and pulsed laser irradiation. Lasers Med. Sci. 10:43-46 (1995) TAPPARO A., SOLDA’ L., BOMBI G.G., ZAMBENEDETTI P., ZATTA P., BERTANI R., CORAIN B., Analytical validation of a general protocol for the preparation of dose-controlled solutions in aluminium toxicology. Analyst 120: 2425-2429. (1995) ZATTA P. 45 Aluminum binds to hyperphosphorylated Tau in Alzheimer's disease: A hypothesis. Medical Hypoth. 44: 169-172. (1995) ZATTA P., ZAMBENEDETTI P. Fisiopatologia dell'alluminio. Le Scienze. 325: 28-35. (1995) ZATTA P., ZAMBENEDETTI P., PIZZIUTI A., PERAZZOLO M. Different effects of aluminium (III) upon carbonic anhydrase and Na +/K+-ATPase. Neurosc. Letters 197: 1-4. (1995) ZATTA P., ZAMBENEDETTI P., MARTURANO M.B., PALUMBO M., NICOLINI M. Effects of Tacrine upon murine neuroblastoma cells J. Neuron. Transm. 102: 113-123. (1995) ZATTA P., ZAMBENEDETTI P., NICOLINI M. La chimica dell’alluminio e i sistemi biologici. Dati certi e questioni aperte. La Chimica e L’industria, 77: 797-802. (1995) (1996) BELTRAMINI, M., BORGHI, E., DI MURO, P., LA MONACA, A., SALVATO, B., SANTINI, C. The use of SAXS in the study of quaternary organisation of giant proteins. J. Molec. Struct. 383, 231-236 (1996). BELTRAMINI, M., BORGHI, E., DI MURO, P., LA MONACA, A., SALVATO, B., SANTINI, C. Functional SAXS study of hemocyanin dioxygen-carrier protein. J. Molec. Struct. 383, 237-240 (1996). BOTEVA, R., FILIPPI, B., SALVATO, B. Evidences for adenine nucleotide binding in the subunits of Neurospora mitochondrial processing peptidase. Arch. Biochem. Biophys. 332, 329-334 (1996). BOTEVA, R., ZLATEVA, T., DOROVSKATORAN, V., VISSER, A.J.W.G., TSANEV, R., SALVATO, B. Dissociation equilibrium of human recombinant interferon gamma. Biochemistry 35, 14825-14830 (1996). BOTEVA, R., SALVATO, B. Catalytic mechanism of mitochondiral processing peptidase: fluorescence studies. Arch. Biochem. Biophys. 332, 323-328 (1996). BUBACCO, L., MAGLIOZZO, R.S., BELTRAMINI, M., PEISACH, J., SALVATO, B. Nitrite reductase activity of deoxy Carcinus maenas hemocyanin: formation of the half-met derivative. Inorg. Chem. 35, 1393-1394 (1996). DE FILIPPIS, V., VASSILIEV, V.B., BELTRAMINI, M., FONTANA, A., SALVATO, B., GAITSKHOKI, V.S. Evidence for the molten globule state of human apo-ceruloplasmin. Biochim. Biophys. Acta 1297, 119-123 (1996). DI MURO, P., ANGELINI, E., CUOMO, V., BELTRAMINI, M., SALVATO, B. A molecular and integrated approach to the respiratory physiology of marine invertebrates; relevance for the study of adaptation to the antarctic environment. Proc. Third Meet. Antarctic Biology, Camerino Univ. Press, Camerino pp. 13-27 (1996). HUBER, M., BUBACCO, L., BELTRAMINI, M., SALVATO, B., ELIAS, H., PEISACH, J., LARSEN, E., HARNUNG, S.E., HAASE, W. Cobalt(II) substituted derivatives of Carcinus maenas hemocyanin: magnetic characterization, magnetooptic and kinetic studies regarding the geometry of the active site. Inorg. Chem. 35, 7482-7492 (1996). VASSILIEV, V.B., KACHURIN, A.M., ROCCO, G.P., BELTRAMINI, M., SALVATO, B., GAITSKHOKI, V.S.. Spectral studies of the active center of ceruloplasmin during the removal and replacement of copper. Biochemistry-Moscow 61 (2), 221-229 (1996) ZAMBENEDETTI P., GIORDANO R., ZATTA P. Identification of lectins' receptors in rat brain. Glycoconjugate J. 13(3): 341-346. (1996) ZATTA P. A new bioluminescent assay for studies of protein G and protein A binding to IgG and IgM. J. Biochem. Biophys. Meth. 32: 7-13. (1996) 46 ZATTA P., ZAMBENEDETTI P., DELL’ANTONE P. Differential uptake of tacrine and physostigmine by murine neuroblastoma cells. Med. Sci. Res. 24: 363-365. (1996) ZATTA P., ZAMBENEDETTI P. Aluminum speciation and morphological differentiation in murine neuroblastoma cells. Biol. Trace Elem. Res. 51: 77-85.96 (1996) ZLATEVA, T., DI MURO, P., SALVATO, B., BELTRAMINI, M. The o-diphenol oxidase activity of arthropod hemocyanin. FEBS lett. 384, 251-254 (1996). (1997) ALZUET, G., BUBACCO, L., CASELLA, L., ROCCO, G.P., SALVATO, B., BELTRAMINI, M. The binding of azide to copper-containing and cobalt-containing forms of hemocyanin from the mediterranean crab Carcinus aestuarii. Eur. J. Biochem. 247, 688-694 (1997). CALISSANO, P., CLEMENTI, F., MELDOLESI, J., MONTECUCCO, C., SALVATO, B., SCHIAFFINO, S. Italian basic and applied research. Science 276 (5320), 1773-1774 (1997). HRISTOVA, R., DOLASHKA, P., STOEVA, S., VOELTER, W., SALVATO, B., GENOV, N. Spectroscopic properties and stability of hemocyanins. Spectroc. Acta 53, (3), 471-478 (1997). MANTAREVA, M., SHOPOVA, M., SPASSOVA, G.,WÖHRLE, D., MULLER, S., JORI, G., RICCHELLI, F. Si(IV)-methoxyethylene-glycol-naphthalocyanine: synthesis and pharmacokinetic and photosensitizing properties in different tumour models. J. Photochem. Photobiol., B:Biol. 40:258-262 (1997) MICHAILOV, N.,PEEVA, M.,ANGELOV, I., WOHRLE, D., MULLER, S., JORI, G., RICCHELLI, F., SHOPOVA, M. Fluence rate effects on photodynamic therapy of B16 pigmented melanoma J. Photochem. Photobiol., B:Biol. 37:154-157 (1997) RICCHELLI,F., SALET,C. and MORENO,G. Distribution properties of photosensitizers in biological membranes Trends Photochem. Photobiol., Vol. 4 : 285-289 (1997). SALET, C., MORENO, G. RICCHELLI, F. Effects of Photofrin a Photodynamic Action on Mitochondrial Respiration and Superoxide Radical Generation Free Radical Res. , 26 : 201-208 (1997 ). SALET, C., MORENO, G. , RICCHELLI, F. and BERNARDI, P. Singlet oxygen produced by photodynamic action causes inactivation o the mitochondrial permeability transition pore J. Biol. Chem., 272, n° 35 : 21938-21943 (1997). VASSILIEV, V.B., KACHURIN, A.M., BELTRAMINI, M., ROCCO, G.P., SALVATO, B, GAITSKHOKI, V.S. Copper depletion/repletion of human ceruloplasmin is followed by the changes in its spectral features and functional properties. J. Inorg. Biochem. 65, 167-174 (1997). ZATTA P., ZAMBENEDETTI P., TOFFOLETTI A., CORVAJA C., CORAIN B. Aluminum(III) induces alterations on the physical state of the erythrocytic membrane: an ESR evaluation. J. Inorg. Biochem. 65: 109-114. (1997) ZATTA P., ZAMBENEDETTI P., GIORDANO R., FAVARATO M. Rabbit spinal cord infactuated upon Al(III)-liposomes intravenous treatment. J. Trace Elem. Med. Biol. 11: 170-172. (1997) ZATTA P. Biological models for the study of aluminum neurotoxicity. Acta Med. Romana 35: 592-600. (1997) (1998) ANGELINI, E., SALVATO, B., DI MURO, P., BELTRAMINI, M. 47 Respiratory pigments of Yoldia eightsi, an antarctic bivalve. Mar. Biol. 131, 15-23 (1998). DAINESE, E., DI MURO, P., BELTRAMINI, M., SALVATO, B., DECKER, H. Subunits composition and allosteric control in Carcinus aestuarii hemocyanin. Eur. J. Biochem. 256, 350-358 (1998). DI NOTO, V., ANGELINI, E., BELTRAMINI, M., DALLA VIA, L., SALVATO, B. Fourier transform infrared attenuated total reflectance spectrometry of hemolymph and hemocyanin in water solutions. Vibrat. Spectr. 18, 1-15 (1998) FAVILLA, R., DEL SIGNORE, F., DAINESE, E., BELTRAMINI, M., SALVATO, B. Dissociation kinetics of hemocyanin from Octopus vulgaris. Biochim. Biophys. Acta 1385, 115-125 (1998). GANDOLFI L., STELLA M.P., ZAMBENEDETTI P., ZATTA P. Aluminum alters intracellular calcium homeostasis. Biochim. Biophys.Acta 1406: 315-320. (1998) NORDIO M., ANDRIANI M., GEROTTO M., MARCHINI P., ZAMBENEDETTI P., ZATTA P. Serum concentration of trace elements during different stages of chronic renal failure.Ital. J. Min. Electrol. Metabol. 12: 81-86. (1998) RICCHELLI, F., GOBBO, S., MORENO, G., SALET, C., BRANCALEON, L. and MAZZINI, A. Photophysical properties of porphyrin planar aggregates in liposomes Eur. J. Biochem. 253 : 760-765 (1998) SALET, C., MORENO, G. RICCHELLI, F. Effects of photodynamic action on respiration in non-phosphorylating mitochondria Arch. Biochem. Biophys. 358 : 257-263 (1998) SALVATO, B., SANTAMARIA, M., BELTRAMINI, M., ALZUET, G., CASELLA, L. The enzymatic properties of Octopus vulgaris hemocyanin: the o-diphenol oxidase activity. Biochemistry 37, 14065-14077 (1998). ZAMBENEDETTI P., GIORDANO R., ZATTA P. Histochemical localization of glycoconjugates on microglial cells in Alzheimer's disease brain samples using Abrus precatorius, Maackia amurensis, Momordica charantia and Sambucus nigra lectins. Exp. Neurol. 153: 167-171. (1998) ZAMBENEDETTI P., GIORDANO R., ZATTA P. Metallothioneins are highly expressed in astrocytes and microcapillaries in Alzheimer's disease brain. J. Chem. Neuroanat. 15: 21-26. (1998) ZATTA P., NICOSIA V., ZAMBENEDETTI P. Evaluation of MAO activities on murine neuroblastoma cells upon acute or chronic treatment with aluminum(III) or tacrine. Neurochem. Intern. 32: 273-279. (1998) ZATTA P., CERVELLIN D., ZAMBENEDETTI P. Effects of aluminum speciation on the morphology of rabbit erythrocytes: a toxicological model. Toxicol. in vitro 12: 287-293. (1998) ZATTA P., ZAMBENEDETTI P., MILACIC R. Aluminum toxicity: the relevant role of the metal speciation. Analusis 26: M54-M58. (1998) ZATTA P. Interdisciplinary contacts. J. Trace MicroprobeTechniques 16: 395-400. (1998) ZLATEVA, T., SANTAGOSTINI, L., BUBACCO, L., CASELLA, L., SALVATO, B., BELTRAMINI, M. Isolation of the met-derivative intermediate in the hydrogen peroxide dismutase activity of deoxygenated Octopus vulgaris hemocyanin. J. Inorg. Biochem. 72, 211-215 (1998). (1999) BELTRAMINI, M., DI MURO, P., FAVILLA, R., LA MONACA, A., MARIANI, P., SALVATO, B., SOLARI, P.L. SAXS investigations on the temperature dependence of the conformation of 5S hemocyanin subunit of Carcinus aestuarii. J. Molec. Struct. 475, 73-82 (1999). 48 DAINESE, E., ANGELUCCI, C. B., VASSILIEV V., BELTRAMINI M., SALVATO B., COZZANI I., SALVATO B., Molecular modification in ceruloplasmin from Wilson disease patients.The Italian Journal of Biochemistry 48: 243-334 (1999). DI NOTO V., DALLA VIA L., ZATTA P. Conformational studies of the Trypsin-Aluminum(III) complex in solution by Raman and Fourier transform infrared attenuated total reflectance spectroscopy. J. Raman Spectr. 30: 209-216. (1999) DOLASKA, P.-A., STOEVA, S., HRISTOVA, R., SCHUETZ, J., BELTRAMINI, M., SALVATO, B., SCHVARTZ, H., VOELTER, W. Structural organisation of hemocyanin from lobster Homarus americanus and spectroscopic studies of the native protein and structural subunits. Curr. Topics Pept. Prot. Res.3 19-36 (1999) GEBAUER, W., STOEVA, S., VOELTER, W., DAINESE, E., SALVATO, B., BELTRAMINI, M., MARKL, J. Hemocyanin subunit organization of the gastropod Rapana thomasiana. Arch. Biochem. Biophys. 372, 128-134 (1999). LANZAVECCHIA S., WADE R. H., GHIRETTI-MAGALDI A., TOGNON G., AND BELLON P.L. A two-exposure technique for ice-embedded samples successfully reconstructs the Chlorocruorin pigment of Sabella Spallanzanii at 2.1nm resolution. Journal of Structural Biology 127, p. 53-63, ( 1999) MESSORI L., ORIOLI P., BANHOLZER V., PAIS I., ZATTA P. Formation of titanium(IV) transferrin by reaction of human serum apotransferrin with titanium complexes. FEBS Lett. 442: 157-161. (1999) RICCHELLI, F., BARBATO, P., MILANI, M., GOBBO, S., SALET, C. MORENO, G. Bis(methyloxyethyleneoxy)silicon Phthalocyanine in Tumour-Bearing Mice J.Photochem. Photobiol. B:Biol. 50 124-128. (1999) RICCHELLI, F., GOBBO, S., MORENO, G. and SALET, C. Changes of the fluidity of mitochondrial membranes induced by the permeability transition Biochemistry 38: 9295-9300 (1999) SALVATO, B., DAINESE, E., COZZANI, I., The Metals: from chemistry to biology. The Italian Journal of Biochemistry 48: 243-334 (1999). SUWALSKY M., UNGERER B., VILLENA F., NORRIS B., CARDENAS H., ZATTA P. Interactions of Al(acac)3 with cell membranes and model phospholipid bilayers. J. Inorg. Biochem. 75: 263-268. (1999) TONELLO F., DUNDON W., SATIN B., MOLINARI M., TOGNON G., GRANDI G., DEL GIUDICE G., RAPPUOLI R. AND MONTECUCCO C. The Helicobacter pYlori neutrophil-activating protein with dodecameric structure. Molecular microbiology 34(2), p. 238-246 , (1999) WÖHRLE, D., MULLER, S., SHOPOVA, M., MANTAREVA, M., SPASSOVA, G.,VIETRI, F., RICCHELLI, F., JORI, G. Effect of delivery system on the pharmacokinetic and phototherapeutic properties of bis(methyloxyethyleneoxy)silicon-phthalocyanine in tumor-bearing mice J. Photochem. Photobiol., B:Biol. 50:124-128 (1999) ZATTA P., TAYLOR A., ZAMBENEDETTI P., MILACIC R., DELL’ANTONE P. Aluminum inhibits the lysosomal proton pump from rat liver. Life Sciences 66: 2261-2266. (1999) ZATTA P., ZAMBENEDETTI P., MILANESE M. Activation of monoamine oxidase type-B by aluminum in rat brain homogenate. NeuroReport 10: 1-4. (1999) ZLATEVA T., BOTEVA R., SALVATO B., TSANEV R., Factors affecting the dissociation and aggregation of human interferon gamma. International-Journal-of-Biological-Macromolecules , 26: 357-362 (1999). (2000) ASCONE, I., SABATUCCI, A., BUBACCO, L., DI MURO, P., SALVATO B. Saccharose solid matrix embedded proteins:a new method for sample preparation for X-ray absorption spectroscopy Eur. Biophys. J. 29: 391-397 (2000). DAINESE, E., SVERGUN, D., BELTRAMINI, M., DI MURO, P., SALVATO, B. 49 Low resolution structure of the proteolytic fragments of the Rapana venosa hemocyanin in solution. Arch. Biochem. Biophys. 373, 154-162 (2000). C. BEGHETTO, C. RENKEN, O. ERIKSSON, G. JORI, P. BERNARDI, F. RICCHELLI Implications of the generation of reactive oxygen species by photoactivated calcein for mitochondrial studies. Eur. J. Biochem. 267:5585-5592 (2000) HOLM, J.K., HEMMINGSEN, L., BUBACCO, L., SALVATO B., BAUER, R., Interaction and coordination geometries for Ag(I) in the two metal sites of hemocyanin. Eur. J. Biochem. 267:1754-1760, (2000). MANZANO, M., COCOLIN, L., CITTERIO, B., CONTE, B., DE BERTOLDI, M., COMI, G., SANTOVITO, G., BELTRAMINI, M., SALVATO, B. Biochemical responses in a Candida famata strain adapted to high copper concentrations. Biometals 13, 251-259 (2000). MOLON, A., DI MURO, P., BUBACCO, L., VASILYEV, V., SALVATO, B., BELTRAMINI, M., CONZE, W., HELLMANN, N., DECKER, H. Molecular heterogeneity of the hemocyanin isolated from the king crab Paralithodes camtschaticae. Eur. J. Biochem. 267, 7046-7057 (2000). PECERE, T., GAZZOLA, M.V., MUCIGNAT, C., PAROLIN, C., VECCHIA F.D., CAVAGGIONI A., BASSO G., DIASPRO A., SALVATO B., CARLI M., PALU G. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Canc. Reser. , 60: 2800-2804, (2000). TONINELLO, A., CLARI, G., MARCON ,M., TOGNON, G. AND ZATTA ,P. Aluminum as an inducer of the mitochondrial permeability transition. J. of Biol. Inorg. Chem. 5,5, p. 612-623 (2000) VALANDRO L., SALVATO B., CAIMMI R., GALZIGNA L. Isomorphism of quasispecies and percolation models. J. Theor. Biol. 202(3): 187-194 (2000) . ZAKHAROVA, E.T., SHAVLOVSKI, M.M., BASS, M.G., GRIDASOVA, A.A., PULINA, M.O., DE FILIPPIS, V., BELTRAMINI, M., DI MURO, P., SALVATO, B, FONTANA, A., VASILYEV, V.B., GAITSKHOKI, V.S. Interaction of lactoferrin with ceruloplasmin. Arch. Biochem. Biophys. 374, 222-228 (2000). ZATTA P., LAIN E., CAGNOLINI C. Effects of Aluminum on Activity of Krebs Cycle Enzymes and Glutamate Dehydrogenase in Rat Brain Homogenate. Eur. J. Biochem. 267: 3049-3055. (2000) (2001) DOLASHKA-ANGELOVA, P., BELTRAMINI, M., DOLASKI-A., SALVATO, B., HRISTOVA, R., VOELTER, W. Carbohydrate composition of Carcinus aestuarii hemocyanins. Arch. Biochem. Biophys. 389, 153-158. (2001) HIDALGO J., ASCHNER M., ZATTA P., VASAK M. Roles of the metallothionein family of proteins in the central nervous system. Brain Res. Bull. 55: 133-146. (2001) GARBISIA S., SARTOR L., BIGGIN S., SALVATO B., BENELLI R., ALBINI A., Tumor Gelatinases and Invasion Inhibited by the Green Tea Flavanol Epigallocatechin-3-Gallate. Cancer, 91: 822-832 (2001). MOCCHEGIANI E., GIACCONI R., CIPRIANOC., MUZZIOLI M., FATTORETTI P., BERTONIFREDDARI C., ISANI G., ZAMBENEDETTI P., ZATTA P. Zinc-bound metallothioneins as potential biological markers of ageing. Brain Res. Bull. (2001) 55: 147-156. (2001) MOLON, A., DI MURO, P., BUBACCO, L., VASILYEV, V., SALVATO, B., BELTRAMINI, M., CONZE, W., HELLMANN, N., DECKER, H. Molecular heterogeneity of the hemocyanin isolated from the king crab Paralithodes camtschaticae. Eur. J. Biochem. 267, 7046-7057 (2000). MORENO, G., POUSSIN, K., RICCHELLI, F., SALET, C. The effects of singlet oxygen produced by photodynamic action on the mitochondrial permeability transition differ in accordance with the localisation of the sensitizer. Arch. Biochem. Biophys. 386: 243-250 (2001) 50 NEGRISOLO E., PALLAVICINI A., BARBATO R., DEWILDE S., GHIRETTI-MAGALDI A., MOENS L., LANFRANCHI G. The evolution of extracellular hemoglobins of annelids, vestimentiferans, and pogonophorans. Journal of Biological Chemistry 276 (28) : 26391-26397 (2001) RICCHELLI, F., CAMERIN, M., BEGHETTO, C. CRISMA, M., MORETTO, V., GOBBO, S. SALVATO, B., SALET, C., MORENO, G. Disaccharide modulation of the mitochondrial membrane fluidity changes induced by the membrane potential IUBMB Life 51: 111-116 (2001) SALVATO, B., CUOMO, V., DI MURO, P., BELTRAMINI, M. The Effects of Environmental Parameters on the Oxygen Consumption of Four Marine Invertebrates: a Comparative Factorial Study. Mar Biol. 138, 659-668 (2001). SABATUCCI, A., ASCONE, I., BUBACCO, L., BELTRAMINI, M., DI MURO P., SALVATO, B Comparison of the X-ray absorption properties of the binuclear active site of molluscan and arthropodan hemocyanins. J. Biol. Inorg. Chem.(2001) SUWALSKY M., UNGERER B., VILLENA F., NORRIS B., CARDENAS H., ZATTA P. Effects of AlCl3 on toad skin, human erythrocytes, and model Brain Res. Bull. 55: 205-210. (2001) COMMUNICATIONS (1973) TALLANDINI L., SALVATO B., JORI C. Foto ossidazione non sensibilizzata del sito attivo dell’emocianina. X Convegno Soc. It. Biofis. Biol. Mol., Padova (1973). TAMBURRO A.M., SALVATO B., ZATTA P. Dicroismo circolare di alcune emocianine. X Convegno Naz. Soc. It. Biofis. Biol. Mcl., Padova (1973). (1974) JORI C., SALVATO B., TALLANDINI L. Photochemical effects associated with the copper absorption band of native hemocyanin from Octopus vulgaris. IV Intern. Workshop on Hemocyanin, Padova (1974). TAMBURRO A.M., SALVATO B., JORI C. The substitution of Cu(I) in hemocyanin with other metals. IV Workshop on Hemocyanin, Padova (1974). GHIRETTI F., SALVATO B., CHIRETTI-MAGALDI A. Subunits from arthropod hemocyanins. IVmt. Workshop on Hemocyanin, Padova (1974). CHIRETTI-MAGALDI A., MILANESI C., SALVATO B. The synthesis of hemocyanin in Carcinus maenas. IV Int. Workshop on Hemocyanin, Padova (1974). CHIRETTI-MAGALDI A., MILANESI C., SALVATO B. The synthesis of hemocyanin in Carcinus maenas. IV Int. Workshop on Hemocyanin, Padova (1974). GHIRETTI-MAGALDI A., SALVATO B, ZATTA P. The action of urea on the hemocyanin from Octopus vulgaris. IV Int. Workshop on Hemocyanin, Padova, (1974). GHIRETTI-MAGALDI A., MAMMI M. Diffrazione ottica sui cristalli endocellularj di emocianina. XI Convegno Naz. Soc. It. Biofis. Biol. Mol., Camerino (1974). SALVATO B. The reaction of cyanide with the hemocyanin of Octopus vulgaris. IV Intern. Workshop on Hemocyanin, Padova (1974). TAMBURRO A.M., SALVATO B., JORI C. The substitution of Cu(I) in hemocyanin with other metals. IV Workshop on Hemocyanin, Padova (1974). (1975) JORI G. Convegno C.N.R. su Fotochimica, Radiochimica e Chimica delle Radiazioni, Chimica dei Radioelementi, Chimica Nucleare (Roma, Italy). Atti del Convegno, pp. 43-58, 1975 51 (1976) JORI G., SALVATO B., TALLANDINI L. “Photooxidative and spectral studies of Octopus vulgaris hemocyanin” 5th International Workshop on Hemocyanin (Malta) , 1976 ZATTA P., VESSEY D.A. Resolution of endogenous lipid intermediates of glycoproteins synthesis in liver microsomes. Fed. Proc. 35: 948. (1976) (1978) ALBERGONI, V., FAVERO, N. Uso della penicillamina nello studio (1Cl metabolismo del rame. Cornun.Congr.Soc. It.Fisiol., Ferrara (1978). CASSINI, A., BONAZZI, L., ALBERGONI, V. Rilevamento del tasso ceruloplasminico nelle popolazioni di due provincie del Veneto. Comun. Congr. Soc. It. Fisiol., Ferrara (1978). GHIRETTI-MAGALDI, A., SALVATO, B., TOGNON, G. Osservazioni sulla struttura quaternaria delle emocianine dei Molluschi. Congr. Soc. It. Biochimica, Urbino (1978). (1979) CASSINI, A., FAZZIN, G., ALBERGONI, V. Livelli di superossididismutasi e di rame eritrocitario e loro correlazioni nel sangue di un campione di individui sani e di epatopatici. Congr. Soc. It. Fisiol., L’Aquila (1979). CASSINI, A., FAZZIN, C., ALBERGONI, V. Tassi di ceruloplasmina e rame plasmatico e loro relazione con i livelli di rame eritrocitario e di SOD nel sangue di un campione di individui sani ed epatopatici. Congr. Soc. It. Fisiol., L’Aquila (1979). REDDI E., JORI G., SALVATO B. “Solvent effect on the hematoporphyrin-sensitized photooxidation of tryptophan” 7th Annual Meeting of the American Society for Photobiology (Monterey, USA). Book of Abstracts, p. 89, 1979 REDDI E., CANNISTRARO G., DENTI G., JORI G., SALVATO B. “Photobleaching of carotenes by direct visible irradiation in chloroform solution” 7th Annual Meeting of the American Society for Photobiology (Monterey, USA). Book of Abstracts, p. 166, 1979 JORI G., CANNISTRARO S., RICCHELLI F., SALVATO B., TALLANDINI L. “A study of the active site topography of hemocyanins by photochemical techniques” EMBO Workshop on Comparative study and recent knowledge on quaternary structure and active sites of oxygen carriers and related proteins (Tours, France). (1980) ROSSI E., VAN DE VORST A., JOEL G. Hematoporphyrin-sensitized photooxidation of indoles in aqueous micellar systems. VIII International Congress of Photobiology, Strasbourg, France (1980). SALVATO B., BELTRAMINI M., RICCHELLI F., ZATTA P. Aspetti cinetici della reazione tra Hc e CN-. II Convegno Nazionale di Chimica Bioinorganica, Atti dei Congresso, Padova, pp. 59-64 (1980). SALVATO B., ZATTA P. The reaction between hemocyanin and CN. Atti FEBS, Jerusalem (1980). SALVATO B., JORI G., CANNISTRARO S. Specific photooxidation of hemocyanin active site promoted by photoexcitation of the peotein legandcopper charge transfer systems. International Conference on oxygen and oxy—radicals in Chemistry and Biology, Austin (Texas), Book of abstracts, pp. 57-58 (1980). JORI C., SALVATO B., CANNISTRARO S. Fotoossidazìone dei sito attivo dell’Hc per fotoeccitazione della banda a trasferimento di carica legando proteina-rame. II Convegno di Chimica Bioinorganica, Padova (1980). 52 REDDI E., RICCHELI F., JORI G. “Interaction of human serum albumin with hematoporphyrin and its Zn 2+- and Fe3+-derivates” 8th International Congress on Photobiology (Strasbourg, France). Book of Abstracts, abstract P-220, 1980 JORI C., SPIKES J.D., STRAIGHT R.C. Photosensitized oxidations within complex biological structures. International Conference on Oxygen and Oxy-radicals in Chemistry and Biology, Austin, Texas (1980). TALLANDINI L., ROSSI F. Emocianina di Carcinus maenas. Caratteristiche fisiologiche e modulazioni allosteriche da parte di effettori naturali. XVII Congr. Naz. Soc. It. Biofis. Biol. Noi., Atti dei Convegno, p. 12 (1980). CASSINI A., TALLANDINI L., ALBERGONI V. La superossidodismutasi negli Invertebrati: Molluschi. Congr. Soc. It. Fisiol., Caserta (1980). TALLANDINI L., CASSINI A., ALBERGONI V. Le superossidodismutasi negli Invertebrati. Anellidi. XXXII Congr. Naz. Soc. It. Fis., Atti dei Congresso, Caserta,p.232 (1980). ZATTA P., SALVATO B. Ricostituzione dell’emocianina di Carcinus maenas. II Convegno Naz. di Chimica Bioinorganica,. Atti dei Convegno, Padova, pp. 65—67 (1980). (1981) ZATTA P. Zn-protein interactions in Carcinus maenas hemocyanin. Acta A.A.A.S., Washington, Abstract (1981). RICCHELLI F., JOEl C., GENOV N., SHOPOVA N. Comparative studies on the conformation of some pro teases. In: Proc. 1 st mt. Conf. Chem. Biotechn. of products, Varna (Bulgaria), September 21-26, (1981). JORI G., REDDI E., SALVATO B. “Evidence for increased photosensitizing efficiency of protein-bound dyes” 9th Annual Meeting of the American Society for Photobiology (Williamsburg, USA). Book of Abstracts, p. 187, 1981 JORI G., REDDI E., ROSSI B., SALVATO B. “Effetto della struttura chimica e dell’ambiente di reazione sul meccanismo e sull’efficienza dell’attività fotosensibilizzatrice di porfirine” V Riunione Società Italiana di Biofisica Pura e Applicata (Perugia, Italy). Book of Abstracts, pp. 4345, 1981 RICCHELLI F., SALVATO B., JORI G. “Studi conformazionali sull’emocianina di Octopus vulgaris negli stati di aggregazione” V Riunione Società Italiana di Biofisica Pura e Applicata (Perugia, Italy). Book of Abstracts, pp. 8485, 1981 (1982) SALVATO B., JORI G. “Mono-and di-phenol oxidase activity of Octopus vulgaris hemocyanin” EMBO Workshop on Structure and Function of Invertebrate Respiratory Proteins (Leeds, United Kingdom). Book of Abstracts, p. 12, 1982 RICCHELLI F., ROSSI E., SALVATO B., JORI G., BANNISTER J.V. “Fluorescence studies on copper/zinc superoxide dismutase from bovine erythrocytes” 3rd International Conference on Superoxide and Superoxide Dismutase (New York, USA). Book of Abstracts, pp. 320-323, 1982 TALLANDINI L., CASSINI A., RICCHELLI F. FILIPPI B. Chemical and spectroscopic characterization of a copper/zinc superoxide dismutase from a marine bivalve mollusc ( Mytilus galloprovincialis ). ibidem , Abs. PI 11. (1983) BERTOLONI G., DALL’ACQUA M., VAZZOLER M., SALVATO B., JORI G. “Bacterial and yeast cells as models for studying the photosensitization by hematoporphyrin” International Symposium on Porphyrins in Tumor Phototherapy (Milano, Italy). Book of Abstracts, pp. 53 37-39, 1983 RICCHELLI F., JORI G., SHOPOVA M., BOTEVA R., GENOV N. “Lanthanide spectral properties as a probe of calcium-binding sites in mesentericopeptidase” 1st International Conference on Bioinorganic Chemistry (Firenze, Italy) , 1983 BACCI M., LINARI R., RICCHELLI F., SALVATO B. About the origin of a novel fluorescence observed in metalloproteins. ibidem , p. 276. ZATTA P., RICCHELLI, F. Metal-protein interaction in Carcinus maenas hemocyanin. ibidem , pp. 155-156. ZATTA P., ZAKIM D. UDP-Glucuronyl-transferase activity in Cancer magister. Abstract FEBS Meeting, Bruxelles (1983). ZATTA P. Contributo deli ‘emocianirta all ‘equilibrio osmotico di Carcinus maenas. Atti Convegno SIBS-SIF— SINU, St. Vincent (1983). (1984) ZATTA P. Interazione Ca2+_Taurina nel cuore di Carcinus maenas. Atti Convegno SIBS-SIF-SINU, 65 (1984). ZATTA P. Sulla partecipazione dell'emocianina all'osmoregolazione del Carcinus maenas. Conv. Unione Zoologica Italiana. Padova, Italy, June 26-30. Boll. Zool. 51: suppl. 117. (1984) ZATTA P., BISMONDO A. Interaction between Taurine and metal ions. FEBS-Meet., Moscow, Russia, XXII-148. (1984) BERTOLONI G., SALVATO B., JORI G. “Photosensitization of microbes by hematoporphyrin” Congresso Annuale della Società Italiana di Fotobiologia (Pavia, Italy). Book of Abstracts, p. 435, 1984 BERTOLONI G. “Studies on the mechanism of cell photosensitization by hematoporphyrin” 9th International Congress on Photobiology (Philadelphia, USA). Photochem. Photobiol. 39:45S, 1984 JORI G., REDDI E., SALVATO B., TOMIO L., COZZANI I. “Liposomes and lipoproteins as efficient carriers of porphyrins to tumor cells in vitro and in vivo” Workshop on Porphyrin Photosensitization (Philadelphia, USA). Book of Abstracts, abstract 27, 1984 RICCHELLI F., GOYAL G.C., GROSSWEINER L.I. Structure and photophysics of HpD-A. Ninth International Congress on Photobiology and 12th Annual Meeting of the American Society for Photobiology Philadelphia , Pennsylvania , U.S.A. , 1-6 July 1984 , Abs TAM-F 2. (1985) RICCHELLI F., JORI G. “Spectroscopic studies on the intraliposomal distribution of porphyrins” International Meeting on Porphyrins as Phototherapeutic Agents for Tumor and Other Diseases (Alghero, Italy). Book of Abstracts, p. 35, 1985 RICCHELLI F., FILIPPI B., GOBBO S., SIMONI E., TALLANDINI L., ZATTA P. Functional and Structural Properties of the 50,000 D Subunit of Octopus vulgaris hemocyanin. Int. Symp. on: Invertebrate Oxygen Carriers. Tutzing-Bavaria, Fed. Rep. Germany, July 29 - August 1, p. 41. (1985) BELTRAMINI M., RICCHELLI F., JORI G., ZIRON L., PAGNAN A. “Interaction of hematoporphyrin with isolated lipoproteins: a spectroscopic study” International Meeting on Porphyrins as Phototherapeutic Agents for Tumor and Other Diseases (Alghero, Italy). Book of Abstracts, p. 37, 1985 BOMBI G.G., CORAIN B., SESTI A.G., ZATTA P. Chemical and Biological Investigations on Aluminum Neurotoxicity. New Trends in Aging Research, Bologna, Italy, November 6-9(CNR-NIH-EURAGE), p. 114. (1985) REDDI E., BELTRAMINI M., RICCHELLI F., JORI G. “Spectroscopic studies on the interaction of hematoporphyrin with liposomes and lipoproteins” 13th Annual Meeting of the American Society for Photobiology (New Orleans, USA). Book of Abstracts, p. 45, 1985 REDDI E., RICCHELLI F., BELTRAMINI M., JORI G. “Interaction of porphyrins with liposomes and lipoproteins” Reunion sur la Photosensibilisation, Socièté Francaise de Photobiologie (Orsay, France). Book of 54 Abstracts, abstract D-7. (1985) TALLANDINI L., SIMONI E., ZATTA P. Interazione lipidi-proteina nell’emocianina di Carciuns maenas. 31 Congr. Naz. SIB, Cleub Ed., Bologna, H 13 (1985). (1986) GENOVA L., GENOV N., BOTEVA R., PRODANOV M., RICCHELLI F., SALVATO B. Superoxide dismutase from Prunus cerasifera. International Symposium and Colloquium School on Activated Oxygen Species in Biological Systems , Varna , Bulgaria , 29 September - 5 October , 1986 , Abs. A 25. GENOVA L., RATKOV A., PEIKOVA S., ANGELOVA M., GENOV N., BOTEVA R., RICCHELLI F. SALVATO B. Purification and characterization of superoxide dismutase from bacterial cell mass of amino acid producers. ibidem, Abs. A 26. SALVATO B., BELTRAMINI M., ALVIGGI M., GIACOMETTI G.M. On the non equivalence of copper ions in the active site of hemocyanin. In: Atti del Congresso Nazionale di Cibernetica e , Lipari 1985 (Chilleni, S. e Frediani, C., eds.) Istituto di Biofisica del CNR, Pisa, pp. 1-6 (1986). BOMBI G., CORAIN B., GIORDANO R., PAGANI D., SESTI A.G., ZATTA P. Chemical and biological investigations on Aluminum neurotoxicity. In: Neuroendocrine system and aging (Vezzadini, Facchini, Labo eds.), Eurage, pp. 253-258 (1986). RICCHELLI F., JORI G. “Fluorescence studies on liposome-bound hematoporphyrin” 1st Congress of the European Society for Photobiology (Grenoble, France). Book of Abstracts, abstract P51, 1986 ZATTA P., DI STEFANO A. Analisi ultrastrutturale delle branchie di quattro specie di crostacei decapodi: Carcinus maenas, Cancer pagurus, Homarus americanus, Austropotamobius pallipes italicus. Conv. Naz. Soc. Ital. Biol. Marina. Cesenatico, Italy , September 9-12. (1986) MILANESI C., ZATTA P. Aspetti ultrastrutturali delle branchie di due specie di crostacei. Conv. Naz. Unione Zoologica Italiana. Roma, Italy, October 6-11, Boll. Zool. 53: suppl., 23. (1986) ZATTA P., CORAIN B., BOMBI G.G., GIORDANO R., STELLA M.P. Toxicity of lipophilic form of aluminum(III). Ann. Meet. Soc. Ecotoxicol. and Environm. Safety, ISISRoma, Italy, November 12-14, p.15. (1986) ZATTA P., FAVARATO M., DI STEFANO A. L'attivita' L- -amminotransferasica nell'ipostress osmotico di Carcinus maenas. Conv. Naz.Soc. Ital. Biochim. Messina, Italy, September 28 - October 1, BT-5 (1986) ZATTA P., FAVARATO M., DI STEFANO A. L'attivita' transaminasica nei crostacei in funzione della salinita'. XVIII Conv. Naz. Soc. Ital. Biol. Marina, Cesenatico, Italy, September 9-12, BM-17. (1986) (1987) PELLERITO L., STOCCO G.C., GIANGUZZA A., DIA G., GIRASOLO M.A, CEFALU R., MANSUETO C., SALVATO B., BELTRAMINI M., TALLANDINI L., RICCHELLI F. Bioinorganic investigations on metallic and organometallic moieties . Incontro su : Chimica dei Sistemi e dei Processi Biologici , Modena , 19-20 Gennaio 1987 Abs. p.1. RICCHELLI F., JORI G. “Porphyrin aggregation in liposomes” 4th International Conference in Chemistry and Biotechnology of Biologically Active Natural Products (Budapest, Hungary), 1987 RICCHELLI F., TOGNON G., JORI G. “Influence of the liposome organization on the fluorescence properties of hematoporphyrin” 2nd Congress of the European Society for Photobiology (Padova, Italy). Book of Abstracts, abstract C181, 1987 RICCHELLI F., TOGNON G., JORI G. “Aggregation processes of hematoporphyrin and hematoporphyrin dimethylester in liposomes” Conference on New Directions in Photodynamic Therapy (Cambridge, Massachusetts, USA), 1987 RICCHELLI F., BELTRAMINI M., SALVATO B. 55 A preliminary conformational study of the copper sites of laccase . Third International Conference on Bioinorganic Chemistry , Noordwijkerhout ,The Netherlands, 6-10 July,1987, Abs. R-20-K. ZATTA P. Catecholamine and serotonin in the hyposmotic stress of Carcinus maenas. Int. Symposium on Transport in Cell and Epithelia. Copenhagen, Denmark, August 16-21. P. C15. (1987) ZATTA P., CORAIN B., GIORDANO R., FAVARATO M., BOMBI G.G. Coordination State and Toxicity of Aluminum. III Int. Conf. on Bioinorg. Chem. Noordwijkerhout,The Netherlands, July 6-10, R-39-0, p.26. (1987) SPEZIALI M., RIZZIO E., ORVINI E., PERAZZOLO M., ZATTA P. Gallium Determination in Different Human Brain Sites. Int. Symp. on Environmental Aspects of Trace Elements. UNESCO Headquarter. Paris , France, December 1-4, p.77. (1987) TALLANDINI L., SALVATO B., BELTRAMINI M., PIAZZESI A., RICCHELLI F. Co(II)-substitution studies on bovine erythrocyte superoxide dismutase. Ibidem , Abs. R-23-K. TALLANDINI L., SALVATO B., BELTRAMINI M., PIAZZESI A. & RICCHELLI F. Active site properties of bovine erythrocyte superoxide dismutase. ibidem , Abs. B-3. (1988) MILANESI C., ZHOU C., RICCHELLI F. JORI G. Ultrastructural studies on the mechanism of photodynamic therapy. 10th International Congress of Photobiology , Jerusalem , Israel , 30 October - 5 November 1988 , Abs. p. 11. CORAIN B., BOMBI G.G., ZATTA P. Aluminum Coordination Compounds in Biological Investigations: Novel Physiopathological Perspectives?. III Hans Wolfgang Nurnberg Memorial Workshop on Toxic Metal Compounds (Interrelation between Chemistry and Biology). Follonica, Italy, April 10-14. (1988) CORAIN B., BOMBI G.G., ZATTA P., PERAZZOLO M. Aluminum Speciation and Biological Effects. I Int. Meet. on Molecular Mechanisms of Metal Toxicity and Cancerogenicity.Urbino,Italy, September 19-22, p.85. (1988) ZATTA P., CORAIN B., BOMBI G.G., PERAZZOLO M. The Role of the Speciation in the Effects of Aluminum(III) on Membranes Stability and on Selected Enzymatic Activities. I Int. Conf. on Alzheimer's Disease and Related Disorders. Las Vegas, NV, USA, September 6-9, p.319. (1988) BOTEVA R., SEVEROV S., GENOV N., BELTRAMINI M., FILIPPI B., RICCHELLI F., TALLANDINI L., TOGNON G., SALVATO B. General characterization of a mollusc gastropod ( Rapana thomasiana ) hemocyanin. Symposium on Invertebrate Dioxygen Carriers , Leuven , Belgium , 23-27 July 1989 , Abs.p.6. (1989) RICCHELLI F., JORI G., MORENO G., VINZENS F., SALET C. Photosensitization of mitochondria by liposome-incorporated protoporphyrin . Third Congress of the European Society of Photobiology , Budapest, Hungary, 27 August - 2 September 1989 Abs. P-93. RICCHELLI F. Photosensitization of subcellular organelles by porphyrins and phthalocyanines . International Conference of Photodynamic Therapy , Sofia, Bulgaria , 3-5 October 1989 , Abs. L. 22 p. 16. ZATTA P., PERAZZOLO M., FAVARATO M., BOMBI G.G., NICOLINI M. Role of the Metal Speciation in the Action of Aluminum(III) Complexes on Plasmatic Membranes. FEBS-Meet. Rome, Italy, July 2-7. (1989) ZATTA P., PERAZZOLO M., FAVARATO M., BOMBI G.G., NICOLINI M. The Effect of Aluminum Speciation on the Rabbit Erythrocytes. IV Int. Conf. on Bioinorganic Chemistry. Cambridge, MA, USA, July 23- 28. J. Inorg. Biochem. 36(3-4), N014. (1989) CORAIN B., BOMBI G.G., NICOLINI M., ZATTA P. Novel Chemical and Biological Models of Aluminum Toxicity Based on Hydrolytically Stable and Diversely Hydrophilic Aluminum Compounds. First Int. Conf. on Aluminum and Health. Orlando, Florida, December 10-13. (1989) BOMBI G.G., CORAIN B., NICOLINI M., ZATTA P. Sviluppo di nuovi modelli chimici e biologici per lo studio della tossicita' dell'alluminio. Congr. Istituto Superiore Sanita', Roma, Italy, September 21, p. 71. (1989) (1990) 56 RICCHELLI F., JORI G., MORENO G., VINZENS F., SALET C. Effect of the carrier on the photosensitizing properties of porphyrins in mitochondria. IX Congresso della Società Italiana di Biofisica Pura e Applicata , Marciana Marina, 12-18 Maggio 1990, Abs. p. 153. CORAIN B., PERAZZOLO M., FONTANA L., BOMBI G.G., TAPPARO A., CORVAJA C., FAVARATO M., ZATTA P., The Effects of Aluminum(III) on the Integrity of Plasmatic Membranes: Relevance to Alzheimer's Disease. II Int. onf. on Alzheimer's Disease and Related Disorders. Toronto, Canada, July 15-20. Neurobiol. of Aging 11(3), 256. (1990) FAVARATO M., PERAZZOLO M., GOBBO S., NICOLINI M., ZATTA P. Effect of Aluminum(III) Speciation on the Permeability of the Blood-BrainBarrier: Possible Implications in Aluminum Intoxication, Dialisys and Dementia. II Int. Conf. on Alzheimer's Disease and Related Disorders. Toronto, Canada, July 15-20. Neurobiol.of Aging, 11(3), 259. (1990) FAVARATO M., MIZZEN C., KRUCK T.P.A., KRISHNAN B., ZATTA P., McLACHLAN D.T. Chromatographic Resolution of Aluminum Binding Components in Human Serum. II Int. Conf. on Alzheimer's Disease and Related Disorder. Toronto, Canada, July 15-20. Neurobiol. of Aging.11(3), 260. (1990) STULTS N.L., ZATTA P., STOCKS N., CORMIER., M.J. CUMMINGS R.D. The Use of the Recombinant Bioluminescent Protein Aequorin for Solid Phase Assays in Microtiter Plates. ASB, Mol. Biol.and The Am. Assoc. Immunol., Joint Meet, New Orleans, June 4-7, 2675. (1990) ZATTA P., FAVARATO M., PERAZZOLO M., FONTANA L., NICOLINI M. Lipophilic Aluminum (III) Complexes Modify the Permeability of the Blood-Brain Barrier. 35o Congr. Soc. Ital. Biochimica, Bari, Italy, September 29 - October 3. Italian Biochemical Soc. Trans. 2(3), 382. (1990) PERAZZOLO M., FONTANA L., FAVARATO M., CORAIN B., BOMBI G.G. TAPPARO A., NICOLINI M., CORVAJA C., ZATTA P. Effects of Aluminum (III) Speciation on Plasmatic membranes. I Int. Symposium on Metal Ions in Biology and Medicine. Reims, France, May 16-19.Trace Elem. in Medicine.7, 62. (1990) ZATTA P., CUMMINGS R.D., SMITH D.F., CORMIER M.J. Applications of recombinant bioluminescent proteins in assays of potential tumor markers. VI Int. Symp. on Bioluminescence and Chemioluminescence. Cambridge ,U.K., September 10-13. (1990) (1991) TRONCHIN M., GOBBO S., RICCHELLI F., JORI G., MORENO G., VINZENS F., SALET C. “Fotoinattivazione di mitocondri sensibilizzata da porfirine” IX Congresso Annuale della Società Italiana di Fotobiologia (Capri, Italy). Book of Abstracts, 1991 RICCHELLI F., JORI G., GOBBO S., MORENO G., VINZENS F., SALET, C. “Comparison between the photosensitizing efficiency of liposome-bound hematoporphyrin and protoporphyrin on isolated mitochondria” 4th Congress of the European Society for Photobiology (Amsterdam, The Netherlands). Book of Abstracts, abstract A-55, p. 77, 1991 RICCHELLI F., JORI G., MORENO G., SALET C. “Mitochondria as models of complex functional systems to study photosensitization by liposomebound porphyrins” International Conference on Photodynamic Therapy and Laser Medicine (Beijing, China). Book of Abstracts, abstract L78, p. 80, 1991 RICCHELLI F., BOTEVA R., SARTOR G.,DECKER H. Proprietà di fluorescenza dell 'emocianina di tarantola ( Eurypelma californicum ). Convegno Annuale della Società Italiana di Fotobiologia , Capri , 10-11 Giugno 1991 . ZATTA P., FACCI L., LEON A., CORAIN B., PERAZZOLO M., FAVARATO M. Role of Metal Speciation in the Effect of Aluminum(III) on Murine Neuroblastoma Cells. IV Hans Wolfgang Nurnberg Memorial Workshop on Toxic Metal Compounds (Interrelation between Chemistry and Biology). Les Diablerets, Switzerland, March 4-8, 67. (1991) FAVARATO M., ZATTA P., NICOLINI M. Serine Proteases: Inhibitory Effect of Aluminum(III): Potential Implications on Alzheimer's Disease. II Int. Springfield Symposium on Advances in Alzheimer Therapy. Springfield, Illinois, May 3-5, P16. (1991) RICCHELLI F., BOTEVA F., SARTOR G., DECKER H. 57 Sonde fluorescenti per lo studio delle variazioni conformazionali del sito attivo tarantola (Eurypelma californicum ). ibidem. dell'emocianina di (1992) BOTEVA R., RICCHELLI F., SARTOR G., DECKER H. Fluorescence probes to study conformational changes of hemocyanin from tarantula (Eurypelma californicum) during oxygenation. International Congress on Invertebrate Dioxygen Carriers, Lunteren, The Netherlands, 12-17 April 1992, Abs. V-P07. RICCHELLI F., JORI G., MORENO G., SALET C. “Porphyrin – photosensitization of mitocondria” International Conference on Photodynamic Therapy and Medical Laser Applications (Milano, Italy). Lasers Med. Sci., 7:252, 1992 RICCHELLI F., DABBENI-SALA F., JORI G., MORENO G., SALET C. “Photosensitization of mitochondria by liposome-bound porphyrins” 11th International Congress on Photobiology (Kyoto, Japan). Book of Abstracts, abstract 147, p. 305, 1992 ZATTA P., TOLLARDO A.M., FAVARATO M., BAZZATO G., MORACCHIELLO P., NICOLINI M. Rheological measurements of whole blood from Alzheimer's disease, dialysis and multinfarctual dementia patients. The Third International Conference on Alzheimer's disease and Related Disorders. Abano, Italy, July 12-17 . Neurobiol. Aging 13 (suppl. 1), 110. (1992) FAVARATO M., ZANONI S., ZATTA P. Unambigous aluminum(III) localization in senile plaque core from Alzheimer's disease. The Third International Conference on Alzheimer's disease and Related Disorders. Abano, Italy, July 12-17. Neurobiol. Aging 13 (suppl. 1), 160. (1992) CORAIN B., NICOLINI M., ZATTA P. Relevance of metal speciation in directing the biological effects of aluminum(III). The Third International Conference on Alzheimer's disease and Related Disorders. Abano, Italy, July 12-17. Neurobiol. Aging 13 (suppl. 1), 372. (1992) ZATTA P. Controversial aspects on aluminum(III) accumulation and compartmentation in Alzheimer's disease. The Third International Conference on Alzheimer's disease and Related Disorders. Abano, Italy, July 12-17. Neurobiol. Aging 13 (suppl. 1), 373. (1992) MATTIELLO G., GEROTTO M., FAVARATO M., LAZZARI F., ZANOBONI V., PILONI M.G. ZATTA P. Microelemental analysis of plasma from Alzheimer's disease and multinfarctual dementia patients. The Third International Conference on Alzheimer's disease and Related Disorders. Abano, Italy, July 12-17. Neurobiol. Aging 13 (suppl. 1), 385. (1992) TISATO F., ZAMBENEDETTI P., CORAIN B., ZATTA P. Modelling the interaction of membrane phospholipid components with Al(III) in water and aprotic solvents: A NMR approach.The Third International onference on Alzheimer's disease and Related Disorders. Abano, Italy, July 12-17. Neurobiol. Aging 13 (suppl. 1), 388. (1992) FAVARATO M., ZANONI S., ZANOTTI A., ZATTA P. Aluminum(III) induces neuronal degeneration in rat brain. The Third International Conference on Alzheimer's disease and Related Disorders. Abano, Italy, July 12-17. Neurobiol. Aging 13 (suppl. 1), 402. (1992) MASIERO S., ZATTA P. Neuritogenic effects of aluminum(III) on murine neuroblastoma cell cultures.The Third International Conference on Alzheimer's disease and Related Disorders. Abano, Italy, July 12-17. Neurobiol. Aging 13 (suppl. 1), 438. (1992) ZANONI S., BORDIN C., FAVARATO M., GIORDANO R., ZATTA P. Glycosylation study of cerebral cortex from Alzheimer's disease patients with lectins. The Third International Conference on Alzheimer's disease and Related Disorders.Abano, Italy, July 12-17. Neurobiol. Aging 13 (suppl. 1), 547. (1992) MIRZABEKOV T., BALLARIN C., SORGATO M.C. NICOLINI M., ZATTA P. Effect of different aluminum compounds on the outer mitochondrial membrane channel, VDAC. The Third International Conference on Alzheimer's disease and Related Disorders. Abano, Italy, July 12-17. Neurobiol. Aging 13 (suppl. 1), 550. (1992) 58 ARSLAN P., BELTRAME M., NICOLINI M., MASIERO S., ZATTA P. Hyaluronic acid reacts with neurofilament proteins in clonal lines of neuroblastoma. Societa' Italiana di Biochimica. Perugia, Italy, September 21-24, F15. (1992) ZATTA P., CANTINI M., MASIERO S., ARSLAN P. Murine neuroblastoma cells: neuritogenic effects produced by Al(III) are related to the metal speciation.5th Congr. Soc. Ital. Neuroscienze.Modena,Italy, December, 1-4. Neurosc. Lett. Suppl. 43. (1992) ZATTA P., ZANONI S., FAVARATO M. The identification of aluminum in the core of mature plaques from Alzheimer's disease.5 th Congr. Soc. Ital. Neuroscienze. Modena, Italy, December 1-4. Neurosc. Lett. Suppl. 43. (1992) (1993) RICCHELLI F., GOBBO S., JORI G., MORENO G., SALET C. “Fotoinattivazione di mitocondri sensibilizzata da ftalocianine” XI Congresso Annuale della Società Italiana di Fotobiologia (Salice Terme, Italy). Book of Abstracts, abstract 13M, 1993 RICCHELLI F., GOBBO S., JORI G., MORENO G., SALET C. “Phthalocyanine-sensitized photoinactivation of mitochondria” 5th Congress of the European Society for Photobiology (Marburg, Germany). Book of Abstracts, abstract P 4, p. 180, 1993 ZATTA P., MORACCHIELLO P., BAZZATO G. Dismorfismo eritrocitario e alluminemia in soggetti uremici. 34 o Congresso Naz. Soc. Naz. Nefrologia, Pisa, Italy, May 18-21.Giornale Italiano di Nefrologia 10, p.81, 282. (1993) ZATTA P., BAZZATO G., MORACCHIELLO P., NICOLINI M., TOLLARDO A.M. Misure reologiche in soggetti uremici sottoposti ad emodialisi extracorporea e CAPD. 34 o Congresso Naz. Soc. Ital. Nefrologia, Pisa, Italy, May 18-21.Giornale Italiano di Nefrologia 10, p. 82, 283. (1993) FAVARATO M., ZANONI S., NICOLINI M., ZATTA P. Positive reaction for A4 protein in the rat brain treated with aluminum maltolate.Fondation Ipsen pour la reserche therapeutique. Colloques medecine et recherche: Amyloid protein precursors in development, aging and Alzheimer's disease. Fondation Ipsen Colloques medecine et recherche. Lyon, France, June 21, p.42.. (1993) (1994) RICCHELLI F. , GOBBO S., JORI G. NIKOLOV P. “Studi spettroscopici sull’interazione di ftalocianine con membrane mitocondriali” Convegno Nazionale Congiunto di Fotobiologia e Fotochimica (Volterra, Italy). Book of Abstracts, abstract 7S, 1994 RICCHELLI F. GOBBO S, TOGNON G. , JORI G. Spectroscopic studies on the interaction of phthalocyanines and porphyrins with lipid membranes: determination of the nature of dye - binding sites in mitochondrial membranes. XII Congresso SIBPA , Palermo 23 - 28 Settembre 1994 . Abs. D-P10. DELL’ANTONE P., BRAGADIN M., ZATTA P. The accumulation of tacrine in acidic compartments of the cell. Intern. Conf: Dementia in Parkinson's Disease. Jerusalem, Israel, March 20-24. Behavioural Neurology 7:11. (1994) ZATTA P., ZAMBENEDETTI P., CORAIN B. Rilevanza della speciazione del metallo sull'interazione tra alluminio(III) e sistemi biologici. V Nation. Conf.:Interazione di metalli e composti con biomolecole. S. Agnello di Sorrento, Italy, April 7-9, p. 45. (1994) BRAGADIN M., DELL’ANTONE P., PALUMBO M., ZATTA P. Tacrine accumulates in the acidic cell compartments: potential therapeutics implication. IV Int. Conf. on Alzheimer's Disease and Related Disorders. Minneapolis, MN, USA, July 29- August 3. Neurobiology and aging 15 S1: P421. (1994) BRAGADIN M., DELL’ANTONE P., PALUMBO M., ZATTA P. Effects of anticholinergic drugs on neuroblastoma cells and isolated lysosomes. XVI Int. Congr. of Biochemistry and Molecular Biology. New Delhi, India, September 19-22, P10-38. (1994) DELL’ANTONE P., BRAGADIN M., ZATTA P. The accumulation of Tacrine, Amantadine and Trihexyphenidyl in acidic compartments of the cell. XII International Congress of Pharmacology, Montreal, Canada, July 23-29, 13.9. (1994) ZATTA P., BRAGADIN M., ZAMBENEDETTI P., DELL’ANTONE P. Activation of acetylcholinesterase by aluminum. Int.Conf.: Molecular mechanisms of enzyme action. Bangalore, India, September 23-25 , P20. (1994) 59 ZATTA P., ZAMBENEDETTI P. The relevance of the metal speciation on the interaction between aluminum and biological systems.III Congr. Naz. A.I.S.E.T.O.V. Modena, Italy, October 28-29 , p. 47 (1994) (1995) RICCHELLI F., GOBBO S, TOGNON G., JORI G., MORENO G., SALET C. “Temperature-induced changes in fluorescence properties as a probe of porphyrin microenvironment in mitochondrial membranes” Congresso Annuale della Società Italiana di Fotobiologia (Bressanone, Italy). Book of Abstracts, p. 19, 1995 RICCHELLI F., GOBBO S., JORI G., SALET C. MORENO G. “The partition of hematoporphyrin in mitochondrial membranes: formation of noncovalent linear dimers” 6th Congress of the European Society for Photobiology (Cambridge, United Kingdom). Book of Abstracts, p. 85, 1995 SHOPOVA M., PEEVA M., MANTAREVA V., MICHAILOV N., STOICHNKOVA N., JORI G., RICCELLI F., WHORLE D., MULLER S. “The significance of sensitizer photophysical properties for the phototherapeutic effect at pigmented melanoma” 6th Congress of the European Society for Photobiology (Cambridge, United Kingdom). Book of Abstracts, p. 91, 1995 ZATTA P., ZAMBENEDETTI P. Attivazione dell'acetilcolinesterasi da parte dell'alluminio. Riun. Soc. Ital. Neuroscienze. Modelli sperimetali in neurologia e psichiatria. Bosisio Parini, Italy, October 15, C7. (1995) (1996) MANTAREVA V., SHOPOVA M., SPASSOVA G., WOHRLE D., MULLER S., JORI G., RICCHELLI F. Pharmacokinetic and photosensitizing properties dependence on the tumor model of cremophor delivered Si(IV) -methoxyethyleneglycol-naphthalocyanine . 12th International Congress on Photobiology , Vienna , Austria , 1-6 -1996, Abs. RICCHELLI F., MILANI M., GOBBO S., TOGNON G., JORI G., MORENO G., SALET C. “Effetti della fotosensibilizzazione con porfirine sul trasporto del Ca 2+ in organelli subcellulari” II Convegno Nazionale Congiunto di Fotobiologia e Fotochimica (Maratea, Italy). Book of Abstracts, abstract S8, p. 42, 1996 AIMO E., DELL’ANDREA E., GAMBILLARA G., MARTINI G., ZAMBENEDETTI P., ZATTA P. I Int. Congr. on mineral waters and soft drinks. Florence, Italy, May 15-17, p. 63. (1996) ZATTA P., ZAMBENEDETTI P. Aluminum speciation and biological effects: implications in human and experimental toxicology. NATO-ASI. Cytotoxic, Mutagenic and Carcinogenic Potential of Heavy Metals Related to Human Environment. Przesieka, Poland, June 15-26, p. 107-108. (1996) ZATTA P., ZAMBENEDETTI P. Al(III) speciation and cell pathology. NATO-AASI. Cytotoxic, Mutagenic and Carcinogenic Potential of Heavy Metals related to Human Environment. Przesieka, Poland,June 15-26, p. 228-229. (1996) ZATTA P. Aspetti storici ed attuali sull'impiego del magnesio in terapia. Conv. Naz.: Il Magnesio: Aspetti biochimici e clinici. Padova, Italy, October 26, p. 3-5. (1996) ZATTA P. Aspetti fisiopatologici dell'ipomagnesemia dell'anziano.IX Congr. Naz. Ordine dei Biologi: Alimenti, Nutrizione e Cosmetici. Grado, Italy, October 10-13, p. 33-34. (1996) (1997) ZATTA P., ZAMBENEDETTI P. The relevance of aluminum(III) speciation in the modulation of the activity of acetylcholinesterase, Na+/K+-ATPase, carbonyc anhydrase and hexokinase. Conf.on Aluminium and Silicon in biology. Keele University, U.K.,February 24-26. (1997) ZATTA P., ZAMBENEDETTI P. Aluminum and Biological systems: Effects on lysosomes. Intern. Symp. on Trace Elements in Humans: New Perspectives. Athens, Greece, October 9-11, P.L.2. (1997) TONINELLO A., ZATTA P. 60 Effect of aluminum on permeability transition of liver mitochondria. 42 o Congr. Naz. Soc. It. Bioch. (SIB). Joint Symposia. Israel Society for Biochemistry and Molecular Biology and Società Italiana di Biologia Sperimentale. Ancona, Italy, p. 247. (1997) MORENO G., SALET C., RICCHELLI F. Effects of Photodynamic Action on Cell Bioenergetics and Calcium Movements 7th Congress of the European Society for Photobiology , Stresa Italy, 8-13 September 1997, Abs. S4 p.13 . BARANYAI P., KATONA Z., RICCHELLI F., JORI G., BITTER I. GROFCSIK A., KUBINYI M., VIDOCZY T. Meso-Tetraarylporphyrins as Molecular Probes of the Microenvironment ibidem, Abs. P107, p. 121. RICCHELLI F., GOBBO S., MORENO G., SALET C., BRANCALEON L., MAZZINI A. Photophysical Properties of Porphyrin Planar Dimers ibidem, Abs. P136, p.128. VISONA’ A., YACOUB A., PAGNAN A., ANGELINI F., CALABRESE G., THIENE., GOBBO S., JORI G. “Prevention of intimal hyperplasia by local delivery of a phthalocyanine photosensitizer” 7th Congress of the European Society of Photobiology (Stresa, Italy). Book of Abstracts, abstract O107, 1997 (1998) BARBATO P., GOBBO S., SALET C., MORENO G., RICCHELLI F. Porphyrin photodynamic action on Ca2+ transport in endoplasmic reticulum. A comparison with mitochondria. Convegno Annuale della Società Italiana di Fotobiologia, Desenzano (BS), 4-5 Aprile 1998.Abs. p.90. RENKEN C., ERIKSSON O., RICCHELLI F., JORI G., BERNARDI P. Photoactivated calcein generates reactive oxygen species (ROS) that are capable of changing Ca2+ accumulation dynamics in isolated rat liver mitochondria. Biophysical Journal 74(2): A382-A382, Part 2 Feb 1998 ZATTA P., GIORDANO R., ZAMBENEDETTI P. Metallothioneins are highly expressed in the astrocytes from Alzheimer's disease brain. VI Int. Conf. on Alzheimer's Disease and Related Disorders. Amsterdam, The Netherlands, 18-23 July. Neurobiol. Aging S50, p209. (1998) ZATTA P., ZAMBENEDETTI P. Effets of aluminum or tacrine on enzymatic activities of MAO A and B in neuroblastoma cells. VI Int. Conf. on Alzheimer's Disease and Related Disorders. Amsterdam, The Netherlands, 18-23 July. Neurobiol. Aging S118, p500 (1998) ZAMBENEDETTI P., ZATTA P. Metallothionein overexpression in astrocytes of Alzheimer's disease subjects. V AISETOV Natl. Conf., Bologna, Italy 25-26 September. (1998) ZATTA P. Aluminum(III) speciation and some biological effects on specific subcellular targets. COST D8 and ESF WORKSHOP on Biological and Medicinal Aspects of Metal Ion speciation. K24. University. JATE, Szeged Hungary, 23-25 August. (1998) ZATTA P., DELL’ANTONE P., ZAMBENEDETTI P. Anticholinesterasic drugs: tacrine, not physostigmine accumulates in intracellular acidic vesicles. VII Congress of the Mediterranean Society of Clinical Pharmacology. Marrakech, October 28-30, C6. (1998) (1999) RICCHELLI F., GOBBO S., MORENO G., SALET C. Changes of membrane fluidity during the mitochondrial permeability transition as probed by hematoporphyrin anisotropy 8th Congress of the European Society for Photobiology, Granada, Spain, 3-8 september 1999 Abs. O77, p.112. BEGHETTO C., GOBBO S., MORENO G., SALET C. RICCHELLI F. Changes of fluidity of mitochondrial membrane induced by the permeability transition. Congresso Annuale della Società Italiana di Fotobiologia, Pavia, 27-29 Maggio 1999. Abs. p. 29. ZATTA P., ZAMBENEDETTI P. 61 Aluminum speciation and biological effects: Potential implications in human pathology. 5th Intern. Symp. on Applied Bioinorganic Chemistry. Corfù, Greece, April 13-17 p. 205. (1999) ANDRIANI M., NORDIO M., ZATTA P. ACE Inhibitors (ACE-I) and angiotensin-II receptors antagonists (Ang II) in kidney protection. I Intern. Congr. on Hypertension: From physiopathology to treatment. Fes, Moroccco, 28-30 October (1999) ZATTA P., ZAMBENEDETTI P. Aluminum neurotoxicity: implications in neurodegenerative diseases. 2nd Intern. Symp. on Trace Elements in Human: New Perspectives. Athens, Greece 7-9 October, P.P.47. (1999) (2000) BEGHETTO C., RENKEN C., ERIKSSON O., JORI G., BERNARDI P., RICCHELLI F. Generation of reactive oxygen species by photoactivated calcein. Implications for mitochondrial studies. Photobiologie 2000, Aix-les-Bains, France, 26-27 May 2000 Abs CAMERIN M., GOBBO S., SALET C., MORENO G., RICCHELLI F. Changes of the fluidity of mitochondrial membranes during opening and resealing of the permeability transition pore. Effects of the medium composition. Photobiologie 2000, Aix-les-Bains, France, 26-27 May 2000 Abs ZAMBENEDETTI P., ZATTA P. Espressione delle metallotioneine nel cervello di mammiferi. VI Nat. Conf. A.I. S.E.T.O.V. Siena, Italy 17-19 February, pp. 19. (2000) ZATTA P. Aluminum neurotoxicity: implications in neurodegenerative diseases. 6th Intern. Symp. on Metal ions in Biology and Medicine. Puerto Rico, 7-10 May. (2000) ZATTA P. Aluminum Neurotoxicity: Implications in Some Neurodegenerative Diseases. VI Intern. Symp. on Metal Ions in Biology and Medicine. San Juan, Puerto Rico, 7-10 May, PL110 (2000) ZATTA P., ANDRIANI M., ZAMBENEDETTI P. Histopathological findings in the brain of uremic patients in dialysis. First International Conference on Metals and the Brain: From Neurochemistryto Neurodegeneration. Padova 20-23 September 2000 J. Alzheimer's Disease vol.2, n.3-4: S1/17. (2000) MOCCHEGGIANI E., GIACCONI R., CIPRIANO C., MUZZIOLI M., BERTONO-FREDDARI C., FATTORETTI P., CARPENE’ E., ZATTA P. Metallothioneins as possible markers pf ageing. First International Conference on Metals and the Brain: From Neurochemistry to Neurodegeneration Padova 20-23 September 2000. J. Alzheimer's Disease vol.2, n.3-4: S2/2. (2000) IBN LKHAYAT-IDRISSI M., MAZZEI P., ZATTA P. In vivo and in vitro effects of Aluminum on acetylcholinesterase. First International Conference on Metals and the Brain: From Neurochemistry to Neurodegeneration Padova 20-23 September 2000. J. Alzheimer's Disease vol.2, n.3-4: S6/9. (2000) ZAMBENEDETTI P., ROSSIPAL E., KARPF E., KLEINERT R., ZATTA P. Expression of metallothionein I+II during human brain development. First International Conference on Metals and the Brain: From Neurochemistry to Neurodegeneration. Padova 20-23 September 2000. J. Alzheimer's Disease vol.2, n.3-4: P7. (2000) CARPENE’ E., ZAMBENEDETTI P., ISANI G., WITTKOWSKI W., ZATTA P. Presence of metallothioneins in bovine hypophysis. First International Conference on Metals and the Brain: From Neurochemistry to Neurodegeneration. Padova 20-23 September 2000. J. Alzheimer's Disease vol.2, n.3-4: P22. (2000) (2001) BEGHETTO C., CAMERIN M., GOBBO S., TOGNON G., SALET C., MORENO G., RICCHELLI F. Perturbation of the mitochondrial membrane structure by disaccharides. Congresso Annuale Società Italiana di Fotobiologia, Pisa, 24-26 Maggio 2001. Abs. p. 45 CAMERIN M., POUSSIN K., GOBBO S., MORENO G., RICCHELLI F., SALET C. The microenvironment of the sensitizer in mitochondrial membranes modulates the photodamage induced by singlet oxygen: a study on the permeability transition. Congresso Annuale Società Italiana di Fotobiologia, Pisa, 24-26 Maggio 2001. Abs. p. 45 BEGHETTO C., CAMERIN M., GOBBO S., TOGNON G., SALET C., MORENO G. RICCHELLI F. 62 Reversibility Properties of the Ca2+-induced Permeability Transition in Mitochondria Suspende in Saline and Sugar-containing Media. IV International Symposium on “Photodynamic Diagnosis and Therapy in Clinical Practice” Bressanone (Italy) 10-13 October 2001, Abs. P18. ZATTA P., ZAMBENEDETTI P. Metallothionein I-II in the developing human brain. SONA: 5 th Soc. Neurosc. Africa Intern. Conf. Nairobi, Kenya 23-27 April. #65. (2001) POLEC K., SZPUNAR J., LOBINSKI R.P., ZAMBENEDETTI P., ZATTA P. Investigation of aluminum binding by neuroblastoma cells by hyphenated techniques. I Intern. Conf. FESTEM, Venice, Italy P.3.14. (2001) ZATTA P., ROSSIPAL E., KARPF E., KLEINERT R., ZAMBENEDETTI P. Expression of metallothioneion I-II in the human brain. I Intern. Conf. FESTEM, Venice, Italy P.3.14. (2001) ZATTA P., ZAMBENEDETTI P. Metallothioneins-I-II expression in some human neurodegenerative disorders, XIV Int. Congr. Polish Pharmacol. Soc. Krakow 10-13 September, pp. 82-83. (2001) ZAMBENEDETTI P., ROSSIPAL E., KLEINERT R., KARPF E., ZATTA P. Metallothionein I-II in the human brain. 3rd Intern. Sympos. On Trace Elements in Humans: New Perspectives. Athens 4-6 October, pp- 75. (2001) Reviews and Books Chapter Salvato B., Beltramini M. Hemocyanins: molecular structure and reactivity of the binuclear copper site. Life Chem. Rep. 5, 249275 (1987). Salvato B., Beltramini M. Hemocyanins: molecular architecture, structure and reactivity of the binuclear copper active site. Life Chem. Rep. 8, 1-47 (1990). Salvato B., Beltramini M. The functional asymmetry of the hemocyanin binuclear copper site. In "Lectures in Bioinorganic Chemistry" (Nicolini, M. and Sindellari, L., eds.) Cortina International Raven Press, New York, pp. 139-157 (1991). Banks W. , A. Kastin A. and Zatta P. The Blood-Brain Barrier in Aluminium Toxicity and Alzheimer's Disease. In: Non-neuronal cells in Alzheimer's disease. Zatta P. and Nicolini M. [Eds]. World Scientific, Singapore, London, pp 1-12. (1995) Pizziuti A., Zambenedetti P. and Zatta P.F. La tossicità dell'alluminio nell'ambiente acquatico. In: Argomenti di Idrobiologia e Acquacoltura. Carpenè E., Isani G. and Serra R. [Eds]. CLUEB, Bologna, pp 249-262. (1995) Ricchelli F. Photophysical properties of porphyrins in biological membranes . J. Photochem. Photobiol. B : Biol. 29 : 109 - 118 (1995). Corain B., Bombi G.G., Tapparo A., Perazzolo M. and Zatta P. Aluminum toxicity and metal speciation: Clear data and open questions. In: Aluminium Chemistry. Corain B., Zatta P., Bombi G.G. and Nicolini M. [Guest eds]. Coord. Chem. Rev. (Special issue) 149: 11-22. (1996) Kiss T., Zatta P. and Corain B. Interaction of aluminum(III) with phosphate-binding sites: biological aspects and implications. In: Aluminium Chemistry. Corain B., Zatta P., Bombi G.G. and Nicolini M. [Guest eds]. Coord. Chem. Rev. (Special issue) 149: 329-346. (1996) Harris W., Berthon G., Day P., Exley C., Flaten T.D., Forbes W., Kiss T, , Orvig C. and Zatta P.F. Speciation of aluminum in biological system. J.Toxicol. Environm. Health 48: 543-568. Lovell M., Ehmann W.D., Markesbery W.R., Melethil S., Swyt C.R. and Zatta P.F. Aluminum biological standards: What are the needs? J.Toxicol. Environm. Health 48: 637-648. (1996) Zatta P. and Zambenedetti P. Aluminum(III) speciation and biological effects: Implications in human and experimental toxicity. In: Cytotoxic, Mutagenic and Carcinogenic Potential Heavy metal Related to Human Environment. (N.D. Hadjiliadis, ed.) NATO-ASI Series, 2. Environment-vol.26, pp. 231-240. (1997) Zatta P. and Zambenedetti P. 63 Aluminum toxicity depends on the metal speciation. In: Aluminium Toxicity in Infants' Health and Disease. Zatta P. and Alfrey A.C., eds. World Sc. Publ. Singapore, p. 40-53. (1997) Zatta P. and Suwalsky M. Aluminium, Membranes and Alzheimer’s disease. In: Aluminium and Alzheimer’s disease. The science that Descibes the Link. C. Exley Ed.. Elsevier Science, Amsterdam, Holland, pp. 279-291. (2001) Hidalgo J, Aschner M., Vasak M., Zatta P. Role of metallothionein family of proteins in the central nervous system. Brain Res. Bull. 55: 133-146. (2001) Mocchegiani E., Giacconi R., Cipriano C., Muzzioli M., Fattoretti P., Bretoni-Freddari C., Isani G., Zambenedetti P., Zatta P. Zinc-bound metallolthionein as potential markers og ageing. Brain Res. Bull. 55: 147-154. (2001) Zatta P., Suwalsky M. Aluminum, Membranes and Alzheimer’s Disease. In: Aluminium and Alzheimer’s disease: The Science that Describes the Link. C. Exley, Ed. Elsevier, Holland, pp. 279-291. (2001) Books and Special Issues GHIRETTI, F. Fisiologia Generale e Animale. Vol. I, UTET, Torino (197).8 GHIRETTI, F., ALBERGONI, V.Fisiologia Generale e Animale.Vol. II, UTET, Torino (1982). GHIRETTI, F., CARIELLO, L. Le biotossine degli organismi marini. Piccin Ed., Padova. GHIRETTI, F., DONATELLI, L., RUSSO, A. (a cura di) Filippo Bottazzi: Leonardo Scienziato. Gianni Editore, Napoli (1986). ZATTA, P. I fitofarmaci in Agricoltura: pensare a scelte strategiche per una agricoltura ambientale. Francisci Editore, Abano, pag. 144 (1986). NICOLINI M., ZATTA P., CORAIN B. (Eds) Aluminum in Chemistry Biology and Medicine. Cortina International, Verona, and Raven Press, New York. pp. 117 . (1991) ZATTA P., SNIDER A. (Eds) Research on age-related phenomena, neurodegeneration and neuropathology. (Book of Abstracts). III International Conference on Alzheimer's Disease and Related Disorders. Neurobiol. aging. vol. 13, Suppl. l (1992) CORAIN B., IQBAL K., NICOLINI M., WINBLAD B., WISNIEWSKI H.M., ZATTA P. (Eds) Alzheimer's disease: Clinical advances and basic research. J. Wiley and sons. Chichester, U.K. (1993) NICOLINI M., CORAIN B., ZATTA P. (Eds.) Alzheimer's Disease and Related Disorders. Oxford, Pergamon Press. pp. 474 (1993) NICOLINI M., ZATTA P. (Eds.) Glycobiology and the Brain. Oxford, UK, Pergamon Press, pp. 293. (1993) NICOLINI M., ZATTA P., CORAIN B. (Eds) Aluminium in Chemistry Biology and Medicine. vol. 2. Life Chemistry Reports (Special issue), Harwood Publ., London, pp. 270. (1994) JORI G., POTTIER R.H., RODGERS M.A.J., TRUSCOTT T.G. (Eds) Photobiology in Medicine NATO ASI Series A272, Plenum Press, New York, pp. 190 (1994) JORI G., MOAN J., STAR W.M. (Eds) Photodynamic Therapy of Cancer , Proc. SPIE 2078, Bellingham, Washington, pp. 564 (1994) JORI G., PERRIA C. (Eds) Photodiagnostic and Phototherapeutic Techniques in Medicine Documento Editoriale, Milano, pp. 165 (1995) NICOLINI M. (Eds) Non-neuronal cells in Alzheimer's disease. World Scientific. Singapore, London pp. 232. (1995) CORAIN B., BOMBI G.G., NICOLINI M., ZATTA P. Aluminum chemistry. Coordination Chemistry Reviews (Special issue) 149, pp. 404. (1996) HONIGSMANN H., JORI G., YOUNG A.R. (Eds) The Fundamental Bases of Phototherapy , OEMF, Milano, pp. 289 (1996) EHRENBERG B., JORI G., MOAN J. (Eds) Photochemotherapy: Photodynamic Therapy and Other Modalities, Proc. SPIE 2625, Bellingham, Washington, pp. 564 (1996) JORI G., KARU T.I. (Eds) Effects of Low-Power Light on Biological Systems II , Proc. SPIE 2929, Bellingham, Washington, (1996) KOSTRON H., JORI G. (Eds) Special issue: Perspectives in Photodynamic Therapy , J. Photochem. Photobiol., B:Biol. 36(2) (1996), Elsevier, Lausanne ZATTA P., ALFREY A.C. (Eds) Aluminum in Infants' Health and Nutrition. World Scientific, Singapore, London, pp. 270. (1997) HONIGSMANN H., KNOBLER R. M., TRAUTINGER F., JORI G. (Eds) Landmarks in Photobiology , OEMF, Milano, pp. 529 (1998) 64 ZATTA P., ZAMBENEDETTI P. Lectins, microglia and Alzheimer' disease. In: Lectins and Pathology (M. Caron and A-P. Sève, eds) Hardwood Acad. Publ., Amsterdam, The Netherlands, pp. 35-49. (1999) ZATTA P., (Ed.) J. Alzheimer's Disease 2 pp. 1-81 (2000) Abstracts of the First Int.Conf. on: "Metals and the Brain: from Neurochemistry to Neurodegeneration" International Projects Bilateral Projects ( Italy / USA ) : a) Biophysical characterisation of copper sites type 3 b) Study of binding sites of metalloproteins through isomorphic substitution Bilateral Projects ( Italy / Germany ) : a) Interaction between metal active site and proteic matrix in copper proteins b) Interaction between metalloproteins and exogenous ligands 1986 –1988 Bilateral Projects ( Italy / France ): Utilization of liposomes as carriers of porphyrins in the tumor phototherapy. Collaboration with Laboratoire de Photophysique Moléculaire, Universitè de Paris Sud, Orsay - France. 1988 -1991 Bilateral Projects ( Italy / France ): Correlation between the subcellular distribution of porphyrins and phthalocyanines and their photophysical and photosensitizing properties. Laboratoire de Biophysique, CNRS et INSERM, Muséum National díHistoire Naturelle, Paris, France. 1988 -1990 Common Research Programs CNR / Bulgaric Academy of Sciences Biochemical, Biophysical and Biotechnological Aspects of Metalloenzymes. Bilateral Projects ( Italy / Germany ) Osmotic properties of hemocyanin. Hanstalt Helgoland, Hamburg, Germany 1990-1994 Bilateral Projects ( Italy / USA ) Lectin chemistry and applications. Department of Biochemistry of University of Athens, Georgia, USA 1991-1993 Common Research Programs CNR / Accademia Bulgara delle Scienze: Functional and Structural Studies on Hemocyanins. 1992-1999 Common Research Programs CNR / Accademia Russa di Medicina Copper Metabolism and Copper Proteins. 1992 -1993 Bilateral Projects ( Italy / France ): Studies of photodynamic effects on the function and structure of cell membranes. Collaboration with Dr. Christian Salet , Laboratoire de Biophysique, CNRS et INSERM, Muséum National d’Histoire Naturelle, Paris, France. 1992-1996 ENEA, Antartica: Molecular Physiology of oxygen in aquatic invertebrates 1994 -1995 Bilateral Projects ( Italy / France ): Photodynamic effects on cell physiology: bioenergetics, calcium transport and cell growth. Laboratoire de Biophysique, Muséum National d’Histoire Naturelle, Paris, France. 1995 –1997 Common Research Programs CNR / Bulgaric Academy of Sciences: Photobiological mechanisms of photodynamic therapy. Institute of Organic Chemistry, BAN, Sofia. 1995-1997 Bilateral Projects ( Italy / UK ) Aluminum neurotoxicity Robens Institute, University of Surrey, Guilford, Surrey, UK (ZattaA.Taylor) 1996 -1999 Bilateral Projects ( Italy / France ): Intracellular photosensitization: photodynamic effects on mitochondria and endoplasmic reticulum. Laboratoire de Biophysique, CNRS et INSERM, Muséum National d’Histoire Naturelle, Paris, France. 1998-2000 Common Research Programs CNR / Bulgaric Academy of Sciences: Mapping the Tryptophan Distribution in Multityrptophan Proteins. 1997-1999 Antartica: Respiratory function in animals adapted to low temperature: Physiological aspects and Biotecnological applications 1999 -2001 Antartica: Development of biosensors utilizing metalloproteins 1999 - 2002 Scientific Cooperation Projects Development of monoclonal antibodies against aluminum. Faculty of Chemistry, University of Concepcion, Chile. Department of Biotechnology, University of Tel Aviv, Israel 2001 Short-term visits Physiological aspects of titanium. Department of Biochemistry, University of Budapest, Hungary 65 2002-2004 Antartica National Projects CNR Strategic Projects: Chemistry of Biological Processes. Chemical Methodologies supplied to the Study of Relevant Biological Phenomena National Project – M.P.I. 40%: Mechanisms of cellular regulation National Project – M.P.I. 40%: Bioinorganic studies on metallic derivatives and organometallic compaunds Co Fin - MURST Supramolecular organisation and functional regulation of oligomeric proteins International Collaborations Biochemistry, University of Budapest, Hungary CYTOPHARM, Palo Alto, California, USA Department of Biochemistry, University of Georgia, Athens, GA, USA Department of Biology, University of Utah at Salt Lake City, USA Departamento de Biologia, Universidad Autonoma de Madrid, Spain Department of Biophysics and Physiology, Yeshiva University, New York (USA) Department of Chemistry, Bulgarian Academy of Sciences, Sofia, Bulgaria Department of Chemistry, Hungarian Academy of Sciences, Budapest, Hungary Department of Chemistry, University of Ohio at Bowling Green, USA Department of Environmental Sciences, …Stefan, Ljubljana, Slovenia Department of Inorganic Chemistry, University of Crete, Greece Department of Inorganic Chemistry, University of Szeged, Szeged, Hungary Department of Inorganic, Metallorganic and Analytical Chemistry and Department of Department of Molecular Biophysics, University of Mainz (GE) Departamento de Quimica, Universidad de Buenos Aires, Argentina Departamento de Quimica, Pontificia Universidad Catolica, Santiago de Chile, Chile Illinois Institute of Technology, Chicago, USA. Institut for Experimental Medicine, RAMS, St. Petersburg Institute of Organic Chemisty and Bulgarian Academy of Sciences. Institut for Physiological Chemistry, University of Tubingen Institute of Zoology, University of Munich, Germany. Laboratoire pour l’Utilisation du Rayonment Electromagnetique, Paris (FR) Laboratoire de Biophysique, CNRS et INSERM, Musèum National d'Histoire Naturelle, Paris, France. Laboratoire de Photophysique Molèculaire , Universitè de Paris-Sud , Orsay, France. Max-Planck Institut für Strahlenchemie, Mülheim/Ruhr, Germany Robens Institute, University of Surrey, Guilford, UK National Collaborations Clinical Medicine Vascular Pathology Unit, Padova. CNR Center of Pharmacological Chemistry, Department of Pharmacological Sciences University of Padova. CNR Center of Quantum Electronic, Institute of Physics, University of Firenze. CNR Center of Quantum Electronic, Polytechnic of Milano. Department of Anatomopathology, General Hospital, Dolo, Venezia. Department of Experimental Biomedical Sciences, University of Padova. Department of General Chemistry, University of Pavia. Department of General Chemistry, University - La Sapienza – Roma. Department of Geriatrics, General Hospital, Dolo, Venezia. Department of Immunology, University of Bologna. Department of Inorganic Chemistry, University of Firenze. Department of Nefrology and hypertension, General Hospital, Dolo, Venezia. Department of Neurosciences, University “Tor Vergata”, Roma. Department of Organic Chemistry, University of Padova. 66 Department of Pharmacology, University of Padova. Department of Biological Chemistry, University of Parma. Department of Physics, University of Ancona. Department of Physics, University of Parma. Department of Physics, University of Palermo. Division of Radiotherapy, Padova general Hospital. Department of Veterinary Pathology University of Padova INFNM – Biophysic section and Department of Physics, University of Parma INFN National Laboratories, Frascati INRCA, Ancona MOLTENI Farmaceutici, Firenze Neurosurgery Clinic, University of Sassari. Organization of International and National Conferences 1992 Colloquia Patavina on Aluminum in Chemistry, Biology and Medicine. Padova. 1992. III International Conference on Alzheimer’s Disease and Related Disorders.Padova. 1994 Colloquia Patavina on Aluminum in Chemistry, Biology and Medicine. Padova. 1997 Magnesium: Biochemistry and Clinical Aspects. Padova 1996 XI International conference on supramolecular organization and functional regulation of invertebrate dioxygen binding proteins. Padova. 2000 Metals and the Brain: From Neurochemistry to Neurodegeneration. Padova Theses 1976/77 Denaturazione con urea dell’emocianina di Carcinus moenas C. Sconfienza. La composizione in aminoacidi delle emocianine: studi comparativi. Mariano Beltramini. 1978/79 Dissociazione dell’Emocianina di Helix pomatia in presenza di urea a bassa concentrazione e ricostituzione dell’Emocianiona di Carcinus maenas con complessi del Cu e del Co Maria Beatrice Sfrappini. 1979/80 Osservazioni comparative sull’ Emocianina di alcuni crostacei Maria Letizia Piccoli. 1980/81 Caratterizzazione dell’Emocianina di Squilla mantis. Tiziana Pianca. Studi comparativi su serin proteasi batteriche tipo subtilisine G. Cappozzo. Influenza di alcuni sali neutri sulla deramazione e ricostituzione dell’emocianina A.Viale. Osservazioni su clorocruorina di Spirographis spallanzani. S. Bonavoglia. 1982/83 Proprietà di fluorescenza di alcuni derivati di emocianina di Octopus vulgaris L. Bellinato. Studi sull’intorno del triptofano in sistemi modello e subtilisine mediante spettroscopia di fluorescenza G. Oradini. Relazioni struttura-funzione in subtilisine M. Manzano. Preparazione di derivati di emocianina di Octopus vulgaris. Marcello Alviggi. 1984/85 Cinetica d iricostituzione dell’emocianina di Carcinus maenas con Cu (I) in presenza di SCN-. Giulio Moriondo. Studi spettroscopici sul derivato a cobalto dell’emocianina di Carcinus maenas. Anna Maria Racioppa. 67 1985/86 Studi spettroscopici sulla interazione dell’ematoporfirina con sistemi microeterogenei D. Stevanin. Derivati ossidati di emocianina. Gianfranco Zilio. Effetto di alcoli, di Ca2+ e di Mg2+ sulla reazione tra emocianina di Carcinus maenas e CN-. Meri Santamaria. 1986/87 Interazione tra emocianina di Octopus vulgaris e CO. Monica Squitieri. Preparazione e caratterizzazione del derivato binucleare a cobalto dell’emocianina di Carcinus maenas. Anna Mazzucato. Facoltà di Scienze, Laurea in Scienze Biologiche Sperimentazione tossicologica con composti idrofili e lipofili di Alluminio (III) Mosé Favarato. 1987/88 Studi spettroscopici e fotocinetici su un derivato dell’ematoporfirina G. Gambaro. Studi spettroscopici su sistemi liposomi-porfirine T. Cavalieri. Modelli chimici e biologici di tossicità da metalli: Tris-(2,4-pentandionato) alluminio (III): Proprietà delle soluzioni acquose, n-ottanoliche e liposomiche e tossicologia sul coniglio (Oryctolagus cuniculus) Maurizio Perazzolo. Modelli chimici e biologici di tossicità da metalli: Ruolo della speciazione dell’alluminio (III) nella sua azione biologica in vivo e in vitro Laura Fontana. Effetti idrolitici di estratti di epatopancreas sull’emocianina di Octopus vulgaris. Renzo Carletti. Cobalto-derivati dell’emocianina di Carcinus maenas 1988/89 Attività pseudo-enzimatiche dell’emocianina di Octopus vulgaris. Graziella Turato. Interazioni di agenti fotosensibilizzanti con modelli di membrana M. Tronchin. 1989/90 Reazione dell’emocianina di Octopus vulgaris con floruro. Reazione tra emocianina di Octopus vulgaris ed azide. Alessandra Giassi. 1991/1992 Il rame e l’ossigeno nel sito attivo delle emocianine. Andrea Maso. Effetti allosterici nella reazione di scambio O2-SCN- dell’emolinfa di molluschi. Enrico Danese. Proprietà di luminescenza dell’emocianina. Anna Favero. Fotosensibilizzazione di mitocondri mediante porfirine legate a liposomi G. Bianco 1992/93 La reazione tra emocianina di Carcinus maenas e nitrito. Carla Bertolli. Coinvolgimento di superossido dismutasi e catalasi nel metabolismo del metanolo nei lieviti. Monica Facco. Studio della luminescenza nella reazione tra CuZnSOD e perossido d’idrogeno. Simona Zanni. Studi strutturali e funzionali sull’eterogeneità molecolare dell’emocianina di Carcinus maenas. Silvia Franzin Modelli chimici e biologici di tossicità da metalli: Studio NMR dell’interazione dell’alluminio(III) con fosfolipidi e membrane eritrocitarie Pamela Zambenedetti 68 Modelli chimici e biologici di tossicità da metalli: Effetto della speciazione dell’alluminio(III) in colture di neuroblastoma murino Sara Masiero Fotosensibilizzazione di mitocondri ad opera di ftalocianine V. Palumbo. 1993/94 Rilevamento in continuo della funzione di saturazione delle’emocianina con ossigeno. Barbagallo Marco. Preparazione e caratterizzazione di un derivato a Cu(II) dell’emocianina di Carcinus maenas. Francesca Gennari. Distribuzione di porfirine e ftalocianine in membrane mitocondriali E. Cumerlato Stiffan. 1994/95 Effetti della fotosensibilizzazione con porfirine sul trasporto del Ca2+ in organelli subcellulari M. Milani. Effetti della Tacrina su cellule di neuroblastoma murino Beatrice Marturano. Caratterizzazione chimico-fisica dell’emolinfa di Carcinus maenas e di Trunculariopsis trunculus Carmelo Calia. 1994/95 Caratterizzazione delle subunità strutturali dell’emocianina di Carcinus maenas Caterina Migale. Reazione dell’azide con derivati ossidati dell’emocianina di Carcinus aestuarii. Annarita Gambalonga Modelli chimici e biologici per lo studio della tossicità dell’alluminio (III): importanza della speciazione del metallo Valeria Bruno 1995/96 Effetti della fotosensibilizzazione con porfirine sul trasporto del Ca2+ in mitocondri P. Barbato Effetti dell’alluminio e tacrina su alcuni sistemi enzimatici Cristiano Cagnolini Reazioni di emocianina di Rapana thomasiana con ossidanti bielettronici. Roberta Rosin 1996/97 Preparazione e caratterizzazione di derivati dell’emocianina di Octopus vulgaris contenenti Co(II). Maria Antonia Lonuzzo. Eterogeneità strutturale e funzionale dell’emocianina di Paralithodes camtschaticae Annamaria Molon Reazione di ossidazione di emocianine con nitriti e ossidi di azoto. Paolo Fioraso Emocianina di Paralithodes camtschaticae: caratteristiche strutturali e funzionali Neurotossicità dello stagno Cristina Cavalieri L’uso del magnesio nella citofilassi e nella pratica clinica Annalisa Coppola Proprietà fotofisiche di aggregati planari di porfirina S. Resoli Aspetti fisiopatologici del magnesio in urologia e nefrologia Stefano Tulio Effetti dell’alluminio sul metabolismo del calcio intracellulare Luisella Gandolfi Effetti dell’alluminio su alcuni enzimi lisosomiali Luca Bordon Effetti dell’alluminio su cellule ematiche Annarita Chimenton Effetti Biologici dell’alluminio e della tacrina su cellule di neuroblastoma murino Valentina Nicosia Aspetti tossici dell’alluminio in pediatria Mason Paola 69 Effetto pro-ossidante dell’alluminio in linfociti umani ed in cellule di neuroblastoma murino Greta Zannini Aspetti fisiopatologici e farmacologici del magnesio nelle emicranie. Laura Sartori 1997/98 Depolimerizzazione di emocianine di molluschi indotta da metalli della prima serie di transizione. Michela Paccagnella Studi preliminari sull’impiego dell’emocianina di Carcinus aestuarii come biosensore per l’ossigeno. Marta Marangotto Caratterizzazione spettroscopica e funzionale della tirosinasi di Streptomyces antibioticus. Maria Chiara Ferrarese Effetti della fotosensibilizzazione con porfirine sul trasporto del Ca 2+ in reticolo endoplasmatico e mitocondri U. Fronzoni Il sovraccumulo di ferro ed alluminio nel morbo di Parkinson: alcuni modelli di studio Margherita Milanese. 1998-99 Variazioni della fluidità delle membrane mitocondriali indotte dalla transizione di permeabilità C. Beghetto Modelli biologici per lo studio di effetti farmacologici della melatonina: possibili implicazioni in alcune patologie neurodegenerative. Stefania Rosan Ruolo della struttura terziaria nel definire la funzionalità dei siti binucleari a rame. Silvia Campello Caratterizzazione strutturale e funzionale dell’emocianina di Penaeus monodon. Carlo Bidona Effetti biochimici dell’alluminio (III) sullle piastrine umane: possibili implicazioni nella malattia di Alzheimer ed in altre patologie neurodegenerative Aurelio Garofano Modelli biochimici e biofisici per lo studio della neurotossicità dell’ Al (III): possibili implicazioni in alcune patologie neurodegenerative Enzo Lain 1999/2000 Effetto dell’ossigeno di singoletto sulla transizione di permeabilità mitocondriale:influenza del microintorno del fotosensibilizzatore M. Camerin Variazioni indotte dal potenziale di membrana sulla fluidità delle membrane mitocondriali e loro modulazione da parte della composizione del mezzo M. Cescon Studio funzionale e strutturale del meccanismo di inibizione dei mercaptani sulla tirosinasi. Andrea Mardegan Studio delle proprietà di proteine respiratorie in matrici solide. Alessandro Cestaro 2001/2002 Proprietà biochimiche della melatonina in vivo e in vitro Paolo Carampin Studi in vivo sulle proprietà biochimiche delle metallotioneine Cristina Di Pisa PhD Theses PhD Theses carried out using space, services and instruments of the CNR Center undre supervision of University Professors members Fisiologia molecolare della superossido dismutasi a rame e zinco in colture cellulari di lieviti (Dott. Paolo Romandini. (Dottorato di Ricerca in Biologia Evoluzionistica III ciclo) Aspetti biofisici e biochimici del sito binucleare a rame di tipo 3 (Dott Meri Santamaria. Dottorato di Ricerca in Biologia Evoluzionistica X ciclo) 70 Struttura di macromolecole biologiche complesse (Dott. Caterina Santini. Dottorato di Ricerca in Biologia Evoluzionistica VII ciclo) Superossido dismutasi a rame e zinco in lieviti e batteri (Dott. Gianfranco Santovito. Dottorato di Ricerca in Biologia Evoluzionistica IX ciclo) Sistemi coinvolti nel trasporto dell’ossigeno: adattamento alle basse temperature nell’ambiente marino (Dott. Elisa Angelini.- Dottorato di Ricerca in Biologia Evoluzionistica X ciclo) Ruolo della struttura terziaria e quaternaria nella funzione di proteina a rame (Dott. Enrico Dainese. Dottorato di Ricerca in Biologia Evoluzionistica X ciclo) Study of evolutionary srategies of hemocyanins, invertebrate oxygene transport proteins (Dott. Annalaura Sabatucci. Dottorato di Ricerca in Biologia Evoluzionistica XII ciclo) Struttura quaternaria delle emoglobine extracellulari degli anellidi: la clorocruorina di Sabella Spallanzani (Gmelin, 1971) (Dott. Alberta Casagrande. Dottorato di Ricerca in Biologia Evoluzionistica XIII ciclo) Fisiologia molecolare di proteine con siti di nucleari (Dott. Stefano Vanin. Dottorato di Ricerca in Biologia Evoluzionistica XIV ciclo) AKNOWLEDGEMENTS I would like to thank Professor M. Beltramini, G. Jori and F. Ricchelli for their contribution for the presentation of this report. I would like to thank also D. Cervellin for the technical support for the preparation of the present booklet and G. Tognon for the e. m. pictures. 71