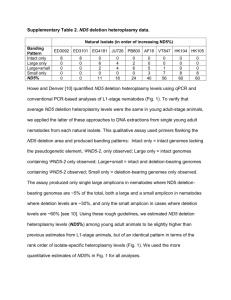
specimens distribution
advertisement

Standard Operating Procedures and Shipping Guidelines for Medical and Pharmaceutical Products: General Information ......................................................................................................................... 2 Quality Management System (QMS) ............................................................................................... 3 Our role in developing a GDP with our Customers ......................................................................... 5 Routing and Station Information ...................................................................................................... 7 Pharmaceutical Products and Vaccines ........................................................................................ 15 Blood Products/Plasma ................................................................................................................. 18 Tissue and Transplant ................................................................................................................... 44 Shipping UN3373 and UN2814 ..................................................................................................... 48 Clinical Trials and Patient Specimen’s .......................................................................................... 58 Cold Chain, Distribution & Warehousing ....................................................................................... 59 Best Practice Solutions for Temperature Ranges and GDP ......................................................... 63 Chain of Custody ........................................................................................................................... 65 Temperature Control/Validation on site ......................................................................................... 76 Temperature Validation in transit................................................................................................... 78 Documentation and Local Regulatory Paperwork ......................................................................... 85 Qualified and Trained Personnel ................................................................................................... 90 MAJAX and Disaster Recovery ..................................................................................................... 92 MHRA/FDA .................................................................................................................................... 95 General Information Purpose: This document is intended to establish detailed procedures and effective command and control protocols for the shipping of Medical and Pharmaceutical products on behalf of CitySprint clients. Objectives: To ensure that proper handling/shipping techniques and transportation requirements, including chain of custody and cold chains are established and maintained for the distribution of various Pharmaceutical Products and Clinical Specimens. CitySprint Medical Express provides specialist tailored transportation services to various pharmaceutical manufacturers, clinical trial organisations, research labs and hospital sites across the UK, Europe and North America, providing direct delivery of Temperature controlled Clinical Specimens; bulk Pharmaceutical Products and Vaccines, Research Samples, Biological Material, Hazardous Goods and Patient Samples from Clinical Trial studies. Quality Management System (QMS) Purpose: CME has developed and will continue to develop an effective internal Quality Management System (QMS). Objectives: To ensure a well designed, structured and organised method of quality assuring the provision of consistent, on-time, validated and effective delivery and clinical logistics services. To provide a means of confirming to regulatory bodies, customers and management that the service provision is in compliance with relevant regulatory and customer standards, and is also a basis whereby improvement in quality systems may be demonstrated. This QMS comprises of the following inter related elements: Good Distribution Practice is the cornerstone of an effective QMS and provides the structure upon which the elements of the quality system can be built. The objective of the GDP is to ensure that the integrity of the product whilst in transit, for the safety of the product for Vaccines, Blood Product and Plasma whilst ensuring clinical specimens for trials and analysis are still at their optimum potency once delivered to the receiving lab for diagnosis. For Transplant the GDP ensures that the tissue or bone marrow reaches it’s destination in the shortest possible time and ensures that the necessary arrangements are in place to act immediately, including the provision of charter aircraft, on-board couriers, arranging special security clearance at the local airport of departure and arrival. Documentation, effective documentation, whether in written or electronic format must be accurate and informative and reviewed on a regular basis to ensure that it remains relevant. It must provide clear instructions on all aspects of the GDP and is there to prevent errors that may occur. This document provides the SOP’s for the distribution of Pharmaceutical, Medical and Clinical Specimens by CME and guidance on the many items described in various Local Government documents for the effective transportation of Pharmaceutical Products and Clinical Specimens. Quality control, Temperature Validation and Traceability, are the most important areas when shipping sensitive Pharmaceutical, Medical and clinical samples. CME must be able to demonstrate quality control and quality monitoring in all aspects of the shipping cycle, from initial discussions with customer to arranging routing and temperature range to final delivery directly to consignee and not the consignees mail facility or loading dock. CME must be able to provide accurate Temperature Validation resources for Pharmaceutical and Blood Products and work with each customer to ensure that we adhere to their audit processes and shipping needs. CME must be able to demonstrate a full audit trail when shipping temperature controlled shipments that require validation; systems are in place with partners such as Sensitech who provide electronic validation kits. Temperature ranges and subsequent routings must be discussed and agreed with the client prior to shipping. Real time tracking must be available 24 * 7, 365 days a year. Tracking information can be viewed on the web at www.citysprint.co.uk, verbal request for updates must be responded to within 60 minutes for all medical shipments (24 * 7, 365 days a year). Resources and Staff Training, CME must have ensured that adequate resources are available to staff at all times to ensure that the GDP is effectively managed and to continually improve its effectiveness and satisfy customer requirements. Staff must be qualified and competent to ensure the work is of the required standard. The physical resources to undertake the work must be suitable to attain the required standards; this will include equipment, consumables, work areas and utilities. Audit, quality audit of the GDP is a planned process of inspection conducted in an independent and detailed way by competent trained individuals to ensure that our procedures and associated quality assurance comply with the principles of our GDP contained within the document. Our independent auditors would be: - Pharmaceutical and Blood Products – MHRA / Clients - Clinical Trials – MHRA / Clients - Clinical Specimens – ISO Auditors - Known Shipper Status - DTI - Airline Shipping and Dangerous Goods – IATA - Routing Set Up – Our Clients Our Internal Auditor is: - Simon Baker - Tel: 1977 Our role in developing a GDP with our Customers Purpose: This document is intended to establish working guidelines in conjunction with our customers for the effective distribution of client samples and pharmaceutical products Objectives: To ratify the many guidelines described in various Local Government documents for the effective transportation of Pharmaceutical Products, Blood Products and Clinical Specimens, whereby information is provided by the following organisations: - WHO - MHRA - DOT/FDA (US) - CDC (US) - USDA (US) - APHIS (Australia) - DEFRA (UK) - IATA/ICAO CitySprint Medical express recognises the importance of effective and secure distribution of client materials, and gives special attention to the integrity of clinical samples, blood, blood products vaccines and other perishable items. When integrating with clients we need to ensure that we are given guidance on the client’s requirements and in turn develop an effective SOP with each customer. There are four fundamentals that we focus on when agreeing the SOP: - Evaluation, CME must be brought into the planning process as early as possible, to ensure that effective line of communication are open, to agree effective SOP’s, to confirm the shipping requirements and temperature validation protocols and to plan the optimal route for each shipment. - Packaging must be qualified and validated to ensure that it can maintain the correct refrigerated or frozen temperature range. Destination and routing will be business critical and will determine the choice of packing used. - Routing, CME will need to clarify routing arrangements and schedules, liaising with its commercial airline partners where the cold chain is utilised in flight and on the ground. Agree tracking points and determine broker arrangements. - Documentation, International shipping requires extra diligence due to veryfing import and export regulations between countries. In particular documentation for US, Chinese and Australian shipments must be 100% prior to shipping. Documentation should always be in order before beginning shipping and explain the purpose of the shipment When agreeing the shipping and cold chain SOP for each client it is imperative that the relevant local country or competent organisations rules and guidelines are adhered to. When shipping Pharmaceuticals and Vaccines in the UK attention must be paid to the “Rules and Guidance for Pharmaceutical Manufacturers and Distributors 2002”, the “Orange Book” in particular; - the “Guidelines on Good Distribution Practice (GDP) OF MEDICINAL PRODUCTS FOR HUMAN USE (94/C 63/03) When shipping whole blood, blood products and plasma in the UK attention must be paid to the “Rules and Guidance for Pharmaceutical Manufacturers and Distributors 2002”, the “Orange Book”. Blood products must follow the Guidelines as per the “Guidelines for the Blood Transfusion Services in the United Kingdom” Routing and Station Information Purpose: This document is intended to clearly demonstrate the global reach of CME and publish the established communication lines globally with our Network of Integrated Partners. Objectives: To provide a clear communication database globally for the provision of Import and Export of Pharmaceutical Products, dry – ice top ups and chain of custody validation. Global Network of over 200 Stations CitySprint Medical Express has developed over time an effective “Integrated Partners” network globally with access to over 200 local country stations and related Clearing Agent Partners. Each consignment forwarded on behalf of our customers will not be grouped or consolidated with any other material; we do not use any of the integrators such as FedEx, UPS, DHL or TNT. Each and every shipment sent through our cool-chain medical network is sent directly to the destination country on it’s own Master Airway Bill, with one of our approved commercial airline partners, such as Virgin Atlantic, BA, Lufthansa, KLM, BMI, American Airlines, Thai, Singapore to name a few. Each consignment will fly directly to the destination country and will be handled individually through customs, where necessary or available a “walk through” clearance will be assured to attempt to clear the consignment within the shortest possible time frame. Where necessary consignments, which are delayed, will be placed in Temperature controlled storage if required, or a dry-ice replenishment will occur for Envirotainers or thermal control units. Cold gel packs will also be replenished where necessary. Once cleared, arrangements are made with the consignee to ensure delivery is made directly to the receiving lab or facility and not the mailroom or loading dock. Prompt Customs Clearance and Correct Packing, Labelling and Paperwork CitySprint Medical Express will handle all elements of the shipment including pre-shipping checks such as: - Check whether local country import permit required - Prepare Advance notification (if required) - Prepare Commercial Invoice, cut MAWB or DGN if required & MSDS - Check that shipment is fit to travel by air as per ICAO/IATA - Check routing and on forwarding to ensure adequate cooling capability - Prepare outer labelling, add required shipping documents & complete NES entry - Check cool-chain requirement against shipping time & organise replenishment alerts, ensure Temptale is correctly validated - Check Packing List attached (if required) - Check if Vaccine Arrival Report attached (if required) - Check Airwaybill is correctly completed In addition to the key elements listed above CitySprint will continually manage the shipments progress through local country customs 24 hours a day. Because CitySprint Medical Express operates 24 hours a day, 365 days a year, all local country customs issues are dealt with in real time either in the UK or in the local country, this ensures that all shipments incur ZERO delay in assisting with clearance. Many of our nearest rivals do not operate on a 24*7 basis, which for time and temperature sensitive shipments can be disastrous, potential clients may incur a 24-hour delay waiting for the UK arm of their shipping agent to handle issues within a different time zone. Through our UK clearing body, “Consortium Sea Company – DGV” we are able to promptly clear all inbound items quickly and effectively. This is evident with the increase of North American Clinical Trial companies electing to use CitySprint to “Collect, Clear and Deliver” within the UK and Europe their Temperature Controlled Vaccines and Investigative Trial drugs. Routing Information Stage 1 The screen shot below captures the initial booking screen. CME utilises booking codes, which are aligned to specific services. The example below is a shipment of vaccines to a consignee in Spain, shipped at 2 - 8c. For instance: HAZ - Hazardous Material MED - Dry Ice shipment (Packing Supplied and Ice supplied by customer) MED+ - Dry Ice shipment (Packing and Ice supplied by CitySprint) MED1 - Temperate Samples MED2 - Samples at 2 –8 (Requires Temperature monitor) MED3 - Infectious Material UN2814 MED4 - UN3373 Diagnostic or Clinical Samples/Specimens Stage 2 Once the shipment is booked, the bar-coded House AWB is created. This contains the delivery details and any special instructions. Stage 3 The shipment is then booked onto a direct flight with a major airline partner. The shipment will be booked directly with the Airline on its own Master Airway bill. We will never consign a shipment to any of the major integrators such as TNT, UPS, DHL or FedEx. Once the shipment has been booked with the Airline we will then “cut” (complete) the MAWB (Master Airway bill). Once complete the shipment is then manifested to the local country delivery station and a serious of pre-alerts are then forwarded by email and by fax. Local Delivery Station Manifest Stage 3 (continued) In addition to the local delivery station manifest, the system also produces a MAWB pre alert, which is also forwarded to the local delivery station. Stage 3 (continued) Accompanied with the Manifest and Pre alert will be any relevant paperwork including: - Scan of MAWB - Transportation Schedule - Commercial invoice - Import Permit if required - MSDS if required - Packing List - Letter of Credit Paperwork (if required) - Advance notification (if required) - VAR if (required) Pharmaceutical Products and Vaccines Purpose: This document is intended to establish working guidelines in conjunction with our customers for the effective distribution of client samples and pharmaceutical products Objectives: To ratify the many guidelines described in various Local Government documents for the effective transportation of Pharmaceutical Products and Clinical Specimens. Information is provided by the following organisations: What are the key elements of the Service Provision? Dedicated Client Customer Support Team CME provides a dedicated pharmaceutical/cool chain team to handle the transportation of Pharmaceutical products and Vaccines. This dedicated team will be able to provide customers advice on global routing options, cool chain integrity solutions and any specific packing requirements. This team will be solely responsible for maintaining the integrity of the cool chain and delivering bespoke global logistics solutions for the client. They will not be working on behalf of any other non medical divisions within CitySprint and will be available 24 hours a day, 365 days a year. The team will also include a senior account manager from our US team based in Atlanta to assist with the North American element of the client requirements. Transportation of Vaccines, General considerations: - Temperature validation must be agreed and implemented into the SOP before shipping begins, agree the temperature validation system and prepare routing schedule. Vaccines should be transported by a secure system using transit containers supplied by the client. The client must ensure that packing materials and procedures have been validated for the purpose to ensure the component surface temperature can be maintained within the prescribed correct ranges during transport. - Monitoring of routine transport temperatures should be performed periodically. - Transit containers should be equilibrated to a component’s storage temperature prior to filling. - Transport containers should be appropriately labelled and should be secure and protect components and samples from damage during transit. - Where prescribed a Vaccine Arrival Report (VAR) should be forwarded. - Where prescribed an Advance Notification should be sent to the consignee with the following information - o Type of Vaccine o Total number of vials and number of doses per vial in the shipment o Number of cartons o Gross weight o Value of Shipment in (US$) o Flight number, ETA and Consignee ETA o House AWB and MAWB umber o Special instructions and collection details Documentation should accompany components in transit to permit their identification. - Transport containers should not be exposed to temperatures beyond the range the range and time for which they have been validated. - Refrigerants should not come into direct contact with the components nor the TempTale. - Dead air space in packaging containers should be minimised. Blood Products/Plasma Purpose: This document is intended to establish working guidelines in conjunction with our customers for the effective distribution of Blood Products and Plasma. Objectives: To ratify the many guidelines described in various Local Government documents for the effective transportation of Blood Products and Plasma. To ensure that all Blood Products and Plasma are What are the key elements of the Service Provision? Dedicated Client Customer Support Team CME provides a dedicated Blood Products and Plasma/cool chain team to handle the transportation of Blood Products and Plasma. This dedicated team will be able to provide customers advice on global routing options, cool chain integrity solutions and any specific packing requirements. This team will be solely responsible for maintaining the integrity of the cool chain and delivering bespoke global logistics solutions for the client. They will not be working on behalf of any other non medical divisions within CitySprint and will be available 24 hours a day, 365 days a year. The team will also include a senior account manager from our US team based in Atlanta to assist with the North American element of the client requirements. Transportation of blood components, General considerations: - Donated blood and blood components should be transported by a secure system using transit containers supplied by the NBS, packing materials and procedures which have been validated for the purpose to ensure the component surface temperature can be maintained within the prescribed correct ranges during transport. - Monitoring of routine transport temperatures should be performed periodically. - Transit containers should be equilibrated to a component’s storage temperature prior to filling. - Transport containers should be appropriately labelled and should be secure and protect components and samples from damage during transit. - Documentation should accompany components in transit to permit their identification. - Transport containers should not be exposed to temperatures beyond the range the range and time for which they have been validated. - Where melting ice is used to achieve an appropriate storage temperature, it should not come into direct contact with the components. Neither should frozen gel packs. - Dead air space in packaging containers should be minimised. NATIONAL BLOOD AUTHORITY Local Courier Work Instructions: 1 INTRODUCTION 2 HEALTH AND SAFETY 3 COURIER SERVICE 4 INVOICING 5 PENALTIES/KEY PERFORMANCE INDICATORS 6 PERFORMANCE REVIEW MEETINGS 7 TRAINING 8 AUDITING NATIONAL BLOOD AUTHORITY Local Courier Work Instructions: 1 INTRODUCTION 1.1 This document defines the services, which are to be delivered by the Courier. It also sets out the respective roles and responsibilities of the Courier and the National Blood Service (NBS) in facilitating and undertaking the delivery of those services. 1.2 Operation procedures relating to the practical application of these instructions by the NBS will be maintained and controlled by the NBS. The Courier will have operational procedures in respect of the processes associated with implementing and undertaking its responsibilities relating to the work instructions. 2 HEALTH & SAFETY 2.1 General Requirements 2.1.1 At the point of collection the driver shall inspect the consignment to ensure that the shipping units show no signs of damage. 2.1.2 The consignment shall be secured on the vehicle to prevent any damage and ensure that it is delivered in the correct condition. The vehicle load compartment doors shall be locked at all times and the drivers doors shall be locked when the vehicle is unattended. 2.1.3 All vehicles must be road worthy with a valid MOT if applicable, road insurance and be fully taxed. 2.1.4 All drivers must adhere to any legislation affecting them in their duty of delivering/collecting as an agent of the National Blood Service (NBS). 2.1.5 At all times the driver must adhere to the site health and safety rules both at collection and delivery points. 2.1.6 No passengers unless engaged by the courier or on accredited Courier work, must be carried whilst on NBS work. 2.1.7 No driver or member of the vehicle crew shall open a package unless authorised to do so by an NBS employee or by a nominated person at the delivery point. 2.1.8 Donations collected from donor sessions will be in a container with an outer thermal container therefore any spillage will be contained within the unit. Samples collected from a donor session will be in boxes and crates and require extra care when loading and unloading from the vehicle. 2.1.9 The collection of blood from Donor sessions will carry a very minor risk of infection should a spillage occur. This is due to a new donor’s blood being included, for which the NBS will not yet have any information. The majority of the blood will be from known donors and will have been tested previously. Products for delivery to Hospitals will consist of tested whole blood, platelets and frozen products and emergency pharmaceutical products. All products will be enclosed in an NBS approved container. The frozen product will also contain a small amount of dry ice pellets and there is a minimal risk of the dry ice giving off CO2 gas as it dissolves all boxes containing dry ice must be labelled as such. In the event of an accident/incident en route, which results in damage or spillage, the courier must follow the emergency information which must be carried on the vehicle at all times. The courier must phone the NBS centre that placed the order and immediately advise them of the incident. 2.1.11Deliveries are to be made where practicable with vehicles containing a bulkhead between the driver and the cargo carrying space. 2.2.1 Blood, which has been collected for the purpose of blood transfusion or for the preparation of blood products, and blood products and any tissues or organs for use in transplants, are not regarded as dangerous goods for carriage. Material which maybe classified as dangerous according to the Carriage of Dangerous Goods (Classification, Packaging and Labelling) Regulations 1996 are set out in Table 1. 2.2.2 Infectious substances carried are in risk group 3 or below and as such are transport category 1. Therefore, the full provisions of the Carriage of Dangerous Goods by Road Regulations 1996 will be required to be complied with when receptacles are over 1 litre or kilogram and the total load is over 20 litres or kilograms. Information regarding the consignment and the statutory declaration will be provided where the individual receptacle exceeds 1 litre or kilogram. 2.2.3 All infectious substances will be packaged in accordance with the requirements of the Carriage of Dangerous Goods (Classification, Packaging and Labelling) Regulations 1996. 2.2.4 In the first instance product to be carried will only be as points 2.1.8 and 2.1.9,any product that falls into the category of Dangerous Goods (as per table 1), will only be carried after both parties agree to it and have consulted with the consignor(s) and both parties dangerous goods advisors. Table 1 Classification of Dangerous Goods for Road Transport (excluding specialised activities e.g. genetic, radioactive materials) Transport Description Risk Types of Materials CDG Streams Apply Stream 1 Samples/units tested H Rare known infected, life saving, Known and confirmed positive bone marrow (e.g. for re-infusion) Infectious for from confirmed infected patients Material similar* HIV, hepatitis or Y Confirmed biohazards ** Repeat donor samples from confirmed donations Stream Samples/units 2aProbable and /possible screening infectious (unconfirmed) tested reactive M to Unconfirmed, screen positive units Y (bio-hazards)** test Unconfirmed, screen positive samples sent for confirmation material Repeat donor screen positive samples from (unconfirmed) donations Stream 2b Samples for diagnostic M/L Diagnostic purposes Specimens patient/donor is known screening, or match greater than 100ml Stream suspected unless Samples of bone marrow typing, to Y tissue or platelet typing, ante-natal be phenotyping, cross infected (see stream 1) 2c Samples for diagnostic Samples of bone marrow typing, Diagnostic purposes Specimens less patient/donor is known screening, than 100ml ## or match suspected unless L to be N tissue or platelet typing, ante-natal phenotyping, cross infected (see stream 1) * HIV, Hepatitis and other agents for which NBS routinely screens and tests blood are hazard Group 3 ** Units to be denatured before transport by road. Obviously not possible when units/specimens where infection is being investigated or tested for. Units recalled from hospital or NBS centre (e.g. NAT pos) may need special handling. ## Exemption from CDG Road Regs available until 31 st December 2003 THE COURIER SERVICE. 3.1 Outline 3.1.1 A courier service shall be provided as per the agreed and documented by both parties, this should outline periods of cover and period when no cover is available. 3.1.2 Jobs will consist of ad hoc short notice, urgent collections and deliveries as required, and pre-planned and notified collections and deliveries. 3.1.3 The type of job can vary from issues from an NBS centre to collection of waste from a remote session’s team. The NBS must communicate what the type of job is when placing the order. 3.1.4 If at any stage the consignment is lost or damaged, the relevant person at the issuing NBS site must be informed immediately, and if required to do so the courier must provide a suitable replacement driver and vehicle at the requested time and site to transport any replacement product. 3.1.5 Biohazard material includes blood, bone, human tissue -all handling and transport of biohazard materials, including blood, bone and human tissue must be secure and the vehicles must be locked when unattended. 3.1.6 Samples from or to hospitals or other centres will be packaged to; The Carriage of Dangerous Goods (Classification, Packaging and Labelling) and use of Transportable Pressure Receptacles Regulations 1996.This point will be amended when the NBS has completed its review of packaging. 3.1.7 The use of motorcycles for the transportation of blood products will be subject to approval by the Authority and can only be used if express permission is obtained from the NBS for each specific instance that one is offered. 3.1.8 The courier must employ all drivers; they must carry ID cards, with photographs and names on them, and be equipped with suitable working mobile phones. 3.1.9 Vehicles exterior must be clean (taking into consideration weather conditions) if it is not it will be rejected and the courier must provide a replacement ASAP, and any service failures following will be deemed to be the courier’s fault. 3.1.10 No sub-contracting of the job may take place without NBS authority. 3.1.11 If at any time the Courier wishes to provide a driver who has not been fully trained on any aspect of the NBS work they must inform NBS at the booking stage to clarify as to whether the job entails doing work for which the driver has not been trained. 3.1.12 The vehicle interior must be clean, with no spillages, odours or dirty equipment in it, the customer has the right to reject any vehicles not up to standard and the courier must provide a replacement up to standard and be liable for any service failures. All drivers must be fully aware of any special local working practices deemed necessary by the NBS, and have all necessary contact names and telephone numbers whilst performing collections/deliveries on behalf of the customer. No animals may be carried in vehicles engaged on NBS work. 3.1.15 At all times the driver must adhere to the site health and safety rules, both at collection and delivery points. 3.1.16 As patient confidentiality is covered by the data protection act, all the couriers employees and agents are bound by this not to reveal any patient details to any body. Therefore no disclosures or information can be released regarding; - The service, its staff or its procedures. - The identity of any patient at any hospitals or other establishments. - The medical condition, tests undergone or treatment of any patient. 3.1.17 The vehicle is not allowed to carry any product other than that issued by the NBS to it. Under no circumstances may it carry product for any other SOE customers whilst it is engaged on work for the NBS. 3.1.18 No courier’s staff shall enter any NBS property without invitation. 3.1.19 The courier must employ for the purposes of these work instructions only such persons as are careful, skilled and experienced in the duties required of them and must ensure that every such person is properly and sufficiently trained and instructed and carries out the services with regard to: - The tasks that person has to perform. - All relevant provisions of the contract. - All relevant rules, policies, procedures and standards of the service. - Fire risks and fire precaution. - The need for those working in the National Health Service to observe the highest standards of hygiene, courtesy and consideration. - The requirements of the Health and Safety at work Act 1974 and other relevant legislation and codes of practice. 3.1.20 The courier shall remove from the premises any of its staffs where the service requests this on the grounds of efficiency or public interest. 3.1.21 The courier shall not advertise or publicly announce that it is supplying a service or undertaking work for the NBS without the prior written consent of the service, such consent not to be unreasonably withheld. 3.2. COMMUNICATION/ORDERING 3.2.1 Order receiving centre phones must be answered within 1 minute. 3.2.2 Only orders from the NBS that quote an accredited order number may be accepted. 3.2.3 If the booking is for a future date it requires confirmation as soon as it has been arranged. 3.2.4 The Courier shall respond i.e. with a suitable vehicle within the locally agreed response period, to each request made by the NBS at all times during each 24 hour period. 3.2.5 The delivery should take place within 15 minutes either side of the requested delivery time. 3.2.6 Whilst carrying out NBS work a non smoking policy shall apply on NBS/NHS sites and in any vehicle engaged on NBS work. 3.2.7 Whilst delivering to hospitals any requests to turn off mobile phones made by the hospital must be obeyed for the duration it takes to make the delivery. 3.2.8 If at any time the delivering vehicle is running late for its delivery the NBS department who placed the order must be informed immediately. 3.2.9 If there any problems at the delivery point the vehicle may not leave until authorised to do so by a NBS employee after resolving the issue. 3.2.10 All delivery notes issued by the NBS must be left at the delivery point. 3.2.11 Any delays above 10 minutes must be communicated to the NBS department who issued the order, to ensure that a fast resolution is achieved. 3.2.12 If upon arrival at a delivery/collection the relevant consignor/consignee can not be located the issue should be immediately communicated to the controlling NBS centre. 3.2.13 If at any time vehicles/drivers/mobile numbers change during a job the customer must be informed to ensure that consignment traceability and process audibility are maintained. 3.2.14 If the vehicle is involved in an accident the NBS centre that made the booking must be informed immediately. 3.3 Collection Point. 3.3.1 Upon arrival at the collection site the driver must report to the nominated collection point, and give their name, vehicle registration and order number issued by NBS and delivery point as nominated by the NBS. 3.3.2 The driver is responsible for case counting on and off all shipping units and ensuring that they agree with the quantities shown on issued paperwork. If it not possible to count the product due to manner in which the customer packages it, the driver must check along with a nominated NBS employee that the correct packages are being loaded and that they are correctly marked up. 3.3.3 The consignment shall be secured on the vehicle to prevent any damage and ensure that it is delivered in the correct condition. 3.3.4 The driver must check all paperwork issued to them against shipping units issued to them, and ensure that they are aware of delivery locations, order of deliveries and required delivery times. 3.3.5 In the case of product being collected from a donor session for delivery to an NBS centre, the blood donations should be counted by number of shipping units and the blood samples by number of individual samples. This count should take place at the collection point and at the delivery point and must be done in conjunction with the NBS employee issuing/receiving the product and the respective quantities agreed before being signed for. 3.4 Delivery Point. 3.4.1 Arrival at delivery point to be within 15 minutes either side of quoted ETA.A 100% achievement is required on this. Allowances will be made for extraordinary condition i.e. major road traffic accidents, weather etc. Reasons to be agreed between both parties. This must be communicated at the time and recorded against any such incident. 3.4.2 Any issues occurring at collection point or delivery point in normal working hours of 0830hrs to 1700hrs Monday to Friday with the exception of Bank Holidays must be reported to the customers transport department who will have placed the order (see contact appendix for local customer details). 3.4.3 Any issues occurring at collection point or delivery point outside normal working, between 1700hrs to 0830hrs Monday to Friday and all day Saturday and Sunday and Bank Holidays must be reported to the customers issues department who placed the order. 3.4.5 During transportation, all packages must be secured to avoid damage and or contamination and carried in a safe and secure manner to prevent accidental damage/loss.... 3.4.6 All deliveries will be made to relevant Blood Banks at each Hospital or to predetermined delivery points as instructed by NBS staff who gave instructions at the time the goods were collected. 3.4.7 All deliveries will be made directly to the nominated delivery points by the quickest route available. No deviations or other third party work to be performed whilst undertaking work for the NBS will be allowed, unless requested to do so by the NBS. 3.4.8 In an event of a Vehicle breakdown, the Courier must ensure the product carried is delivered by alternative arrangements within the time scales and conditions agreed. 3.4.9 In the event of an accident/incident en route, which results in damage or spillage, the courier should not attempt to stem the spillage or clean it up at the roadside. The courier must phone NBS Issuing centre immediately and advise them of the incident. 3.4.10 The NBS must be notified immediately of any disruption/delay to the delivery service. 3.4.11 Upon return to the NBS centre arrangements will be made to remove and dispose of the consignment. 3.4.12 Within 48 hours of the event, you should notify NBS in writing, with a full explanation of the incident. INVOICING 4.1 All invoices will be sent to designated member of National Blood Service staff at the location which has issued the work. 4.2 Each job must show order number, name of person booking the job (first and second name), collection point, delivery point, collection date and time and if more than one delivery was on this job they must be grouped together, and the actual delivery date and time. 4.3The-delivery point format should show hospital name and location by town or in the case of central London by postcode. 4.4.The invoice should break down by collection site, delivery site, job type, date, time and order number. 7 PERFORMANCE REVIEWS. 7.1 Regular review meetings will occur on at an agreed frequency. All aspects of the provision of service 7.2 Any variation to these local instructions must have the agreement of the NBS National transport and Logistics Resource Planning Manger. 7.3 If it is found that problems are taking place at a local level and an immediate solution is not forthcoming; the NBS has the right to call a local meeting at short notice to seek to rectify the issues. 7.4 Any complaints raised by the NBS regarding the provision of service will have to be investigated by the courier and responded to within 3 working days. 8 TRAINING. 8.1 All personnel involved on NBS work to be trained to agreed standards. 8.2 Personnel at the Couriers sites responsible for operation of service to visit local sites and meet NBS staff. 8.3 The Couriers staff need to be familiar with their local sites delivery points and routes from NBS centre to sites. 9 AUDITING. 9.1 The Courier must maintain a list of all drivers trained to do NBS work; this list must be kept updated. 9.2 Trained list to be in a modular format, to allow training on fresh work instructions as issued. 9.3 The NBS has the right to check this list to ensure that drivers doing work on their behalf are fully trained. 9.4 Vehicle service records should be available to ensure that checks can be made on their suitability for the job. On behalf of the National Blood Service Name in capitals Job title in capitals Signature Date On behalf of CitySprint (UK) Ltd Name in capitals Job title in capitals Signature Date NBS – Transport Contact Numbers Transport Contact Numbers. Centre Name Role Mobile Phone Fax 0121253 4166 0771144 (377) 7648 0121253 4169 0121253 4166 0771144 (377) 7528 0121253 4169 0121253 4167 0771144 (377) 7688 0121253 4149 0121253 4166 0771144 (377) 7519 0121253 4149 0127730 6019 0776428 (377) 0928 0127730 6087 Desk Phone Birmingham Alistair Lovie Transport CoOrdinator Birmingham David Mitchell Transport Administrator Birmingham Neville Robinson Transport and Logistics Manager Birmingham David Smith Assistant Transport and Logistics Manager Brentwood Jill Broughton Transport and Logistics Manager Brentwood Gill Harding Clerical 0127730 6023 0127730 6087 Assistant Brentwood Brentwood Stephen Transport Co- Oakham ordinator Brian Payne Assistant 0127730 6072 0776428 (377) 0644 0127730 6087 0127730 6090 0771144 (377) 7220 0127730 6087 0117991 2080 0771144 (377) 7507 Transport and Logistics Manager Bristol Andy Chapman Assistant Transport and Logistics Manager Bristol Carole Harbridge Transport 0117991 2082 Administrator Bristol Cambridge 0117991 2081 Martin Harris Transport and Also covers Logistics Plymouth Manager Alan Hawkes Assistant 01223 246 542 Jill Broughton Transport and Non TLM Brentwood Logistics Featurenet is also TLM for Manager 0771144 (377) 7063 01223 413 782 Non Featurenet Cambridge. Colindale Pearse Allen Transport & 0208258 2934 0771144 (377) 7548 0208258 2897 0771144 (377) 7054 Logistics Manager Colindale Bill McKeown Assistant Transport & Logistics Manager Colindale Julie Taylor Transport Co- 0208258 2990 ordinator Lancaster Colin Richardson Assistant Terry Cain TLM Transport and Manchester is Logistics also TLM for Manager 0152430 6204 0780890 (377) 6306 0152430 7361 Lancaster Leeds Gemma Holroyd Transport 0113214 8633 0113214 8700 Administrator Leeds Chris Thyer Transport and 0113214 8760 0776428 (377) 0895 0113214 8700 0113214 8633 0776428 (377) 0609 0113214 8700 Logistics Manager Leeds Wayne Young Assistant Transport and Logistics Manager Liverpool Ed Bond 0776428 (377) 0307 Senior Resource Planning Data Analyst Liverpool Ged Caswell 0776428 (377) 0771 National 01925 724369 01925 791612 Resource Non Featurenet Non Featurenet Planning Number Number Manager Liverpool Phil Loy Transport & 0151551 8807 0776428 (377) 0987 0151551 8804 0771144 (377) 7376 0161251 4282 0776428 (377) 0786 0161 253 1211 Logistics Manager Liverpool Peter Smathers Assistant Transport and Logistics Manager Manchester Larry Bannon National Fleet Services Manager Manchester Jeff Brandon Fleet Engineer 0161251 1205 0771144 (377) 7273 0161 253 1211 Manchester Terry Cain Transport and 0161251 4248 0771144 (377) 7375 0161251 1222 0161251 4248 0772027 (377) 5319 0161253 1222 0161251 4397 0776428 (377) 0145 0161251 4350 Logistics Manager Manchester Mark Hodgson Assistant Transport and Logistics Manager Manchester Ruth Hopwood PA to National Transport Operations Manager (North) Manchester John Lowden Fleet Controller 0161253 1206 Manchester Avril Marshall Fleet 0161251 4282 0776428 (377) 0163 0161 253 1211 0161 253 1211 Operations Assistant Manchester Andrew O'Brien National 0161251 4397 0780890 (377) 6463 0161251 4350 0191219 4445 0776428 (377) 0960 0191219 4597 0191219 4445 0776428 (377) 0662 0191219 4597 0186544 7924 0776428 (377) 0794 0186544 7924 0186544 0018 0776428 (377) 0772 0186544 0053 Transport Operations Manager (North) Newcastle David Lowe Transport and Logistics Manager Newcastle Duncan Wright Assistant Transport and Logistics Manager Oxford Brian Mitchell Assistant Pearse Allen Transport and TLM Colindale is Logistics also TLM for Manager Oxford Oxford John Hilton MBE National Science Park MILT, whilst Transport John is on AFC Operations secondment Manager Geoff Thorngate (South) at Southampton is covering his role. Plymouth See Bristol Sheffield Ian Easton Transport and 0114203 4822 0771144 (377) 7337 0114203 4822 0114203 4853 0771144 (377) 7325 0114203 4822 Logistics Manager Sheffield Stephen Firth Assistant Transport and Logistics Manager Sheffield Pat Peckett Transport 0114203 4853 0114203 4822 0238029 6796 0238029 6779 Administrator Southampton Carol Baker Transport Administrator Southampton Mel Callaway Assistant Whilst Geoff Transport and Thorngate is Logistics covering for Manager 0238029 6744 0771144 (377) 7541 0238029 6779 0238029 6745 0771144 (377) 7511 0238029 6779 0208258 8389 0771144 (377) 7086 0208258 8539 John Hilton,Mel Callaway is acting TLM at Southampton. Southampton Geoff Thorngate Transport and Also is covering Logistics for John Hilton Manager whilst he is on Southampton AFC, Mel Callaway is covering TLM role at Southampton. Tooting Chris Appleby Assitant Transport and Logistics Manager Tooting Jackie Butler Transport and 0208258 8385 0771144 (377) 7087 0208258 8539 Logistics Manager Tooting Philip Holden Transport Co- 0208258 8580 0208258 8539 0208258 8579 0208258 8539 Ordinator Tooting Susan Holland Transport Administrator Watford Geoff Cotter General 0192348 6848 0771815 (377) 5200 0192348 6802 Manager Transport Watford Sarah Angus PA to General Manager Transport 0192348 6809 0192348 6802 NBS Maps and Directions Tissue and Transplant CME regularly transport Tissue and Bone Marrow for Transplant. This specialised service is maintained by Andrew Turner, ext 1588, mobile 07989 857 816. All tissue or marrow must be hand delivered from pick up to final delivery and OBC couriers are available 24*7. Airline tickets are booked utilising the company credit card (see Andy Turner – 07989 857 816). Patient specimens must not be passed through airport X-rays or Metal Detecting equipment. A prior notice of indented flight must be forwarded to BAA security who will arrange for the courier to be hand searched. If flying from City Airport all arrangements must be made through Adam Tally (020 7646 0088) (Security Manager) who will arrange the prior notice paperwork. City Airport are more flexible than LHR, where possible arrange OBC flights from City Airport Piston Engine, Jet and Helicopter charters are arranged at City Airport through: London City Airport Jet Centre Royal Docks London, E16 2PJ Telephone: 020 7646 0400 Fax: 020 7646 0450 E-mail: jetcentre@lcy.co.uk SITA LCYGAXH Frequency: 131.475 Call sign "Jet Centre Ops" Attached are sample documents that must travel with the shipment. Fax pre alert: Andrew Turner Business Development Manager CitySprint Transplant 58-62 Scrutton Street London, EC2A 4PH CitySprint Transplant 020 7880 1121 Direct Line: Mobile: Direct Fax: URGENT PRE ALERT +44 (0) 20 7880 1588 +44 (0) 7989 857 816 +44 (0) 20 7880 1991 Email: aturner@citysprint.co.uk To: Lisa Hall/ Adam Tally - LCY Fax: From: Andrew Turner Date: Re: Shipment of Bone Marrow Pages: Urgent For Review Please Comment Andy Turner 020 7646 6167 International Sales Director CitySprint International 22nd November 2005 58-62 Scrutton Street London, EC2A 4PH 3 Direct Line: +44 (0) 20 7880 1590 Mobile: +44 (0) 7989 855 351 Please Reply Direct Fax: +44 (0) 20 7880 1991 Email: aturner@citysprint.co.uk Dear Lisa, As discussed the job is now a definite go, please find enclosed the letter of exemption for the shipment that must be hand carried on the BA8706, also attached is the booking itinerary for Mr Marcus Kelly. The samples are bone marrow cells for a patient that is suffering from Leukaemia. They cannot be consigned to the cargo hold as this renders the cells ineffective for treatment. The samples can not be x-rayed as this will kill the cells newly cured cells. The transportation of these samples by hand is patient critical; we appreciate your assistant with these. Regards Andy Direct Line: Mobile: +44 (0) 20 7880 1588 +44 (0) 7989 857 816 Commercial Invoice: Commercial Invoice 23rd November 2005 From: To: Dr Amanda Curran Dr Joanna Mountford Clinical Trial Manager The Royal Infirmary Dept of Gene and Cell Therapy Alexandra Parade National Heart and Lung Institute Glasgow Emmanuel Kaye Building G31 2ER Manresa Road London SW3 6LR Full Description of Contents: Covering 3 million bone marrow cells labelled with Indium-111 with an estimated dose of 10 Mbq x 4 aliquots. Bone Marrow cells for Leukaemia patient undergoing life saving therapy. The Cells are packed in 1 approved fibreboard shipper specifically designed for shipping bone marrow samples. This package conforms to the conditions and limitations specified in section 10.5.9, Excepted Packages of the IATA Dangerous Goods Regulations 47th Edition. This package is also exempt from being x-rayed as per the NASP regulations, chapter 18, Annex F, Item 5, which in summary states that: “ bonafide consignments of life saving nature, other essential medical supplies, human organs, blood plasma” are exempt from being x-rayed. This shipment must not travel in the cargo hold because the pressure and temperature will destroy the cells rendering them ineffective for the patient’s treatment. These samples are being hand carried by Mr Marcus Kelly on flight number BA8706. Mr Kelly’s passport number is 029040972, Mr Kelly’s ticket number is ZWXFTX, the security seal number on the shipping box is X25B. The material does not come from a facility where work with exotic viruses affecting livestock and avian species is conducted. The material is not recombinant. I hereby certify that the contents of this consignment are fully and accurately described above by the proper shipping name and are classified, packed, marked, and labelled, and in proper condition for carriage by air according to applicable national governmental regulations, IATA and DFT regulations Certified true and correct. Dr Amanda Curran Royal Brompton Hospital Shipping UN3373 and UN2814 Purpose: This document is intended to establish working guidelines in conjunction with our customers for the effective distribution of client samples and pharmaceutical products Objectives: To ratify the many guidelines described in various Local Government documents for the effective transportation of Pharmaceutical Products and Clinical Specimens. Information is provided by the following organisations: CitySprint Medical Express FACT SHEET - Shipping Biological Materials & Dry Ice The International Air Transport Association (IATA) and many local Government Agencies, including DEFRA (UK), ADR (UK), Centers for Disease Control and Prevention (CDC - US), the Department of Transportation (DOT - US) have requirements regarding shipment of hazardous materials, which are termed, “dangerous goods". Dangerous goods are articles or substances which are capable of posing a risk to health, safety, property or the environment. Infectious substances, diagnostic specimens, genetically modified microorganisms and dry ice are listed as dangerous goods and must be packaged and shipped accordingly. Implementation of the IATA requirements ensures compliance with all of the other agencies’ requirements. IATA requirements are updated annually, are complex and may be non-intuitive. This fact sheet provides only an overview of the current requirements regarding training, packaging, labeling and transportation regulations. You will also need to know UN codes, specific packing instructions and other information from the IATA Dangerous Goods Regulations Manual to properly prepare your shipment. 2005 Regulatory Update Shipping regulations have changed significantly in 2005. The major updates include: Infectious agents and diagnostic specimens have been classified into lists known as “Category A agents” and “Category B agents.” - Category A agents are infectious materials that have the potential to cause serious/life threatening disease or disability. - Category B agents are generally diagnostic specimens which pose a low risk during transportation. - Diagnostic and clinical specimens must be labeled with UN3373 and be marked “Diagnostic Specimen” or “Clinical Specimen.” - Airway bills for diagnostic specimens will need to include the text “Diagnostic Specimen” or “Clinical Specimen,” and the packaging will require the UN3373 label. Training Requirements IATA (by air) and ADR (by road) training is required for all CME personnel who: - Prepare shipping documentation - Mark and label packages - Repack items that are received in poor or damaged condition - Accept packages - Supervise transport of packages. Training is required initially and must be refreshed within 24 months of the previous training. The training will describe the shipper’s responsibilities and provides the necessary guidelines and references to ensure the safe and compliant transport of dangerous goods. Similar training is also required for authorization to ship other dangerous goods, including chemicals and radioactive materials. IATA Shipping Terms INFECTIOUS SUBSTANCES are class 6.2 Dangerous Goods. IATA defines infectious substances as materials known or reasonably expected to contain pathogens. Pathogens are defined as microorganisms (including bacteria, viruses, rickettsiae, parasites, or fungi) and other agents such as prions, which have the potential to cause disease upon exposure to humans or animals. Infectious substances are assigned to UN classes based on the following definitions: - Category A, is an infectious substance which is transported in a form that is capable of causing permanent disability, life threatening or fatal disease in humans or animals. Category A infectious substances require Packaging Instruction 602 and are assigned to either UN2814 (infectious substances affecting humans) or UN2900 (infectious substances affecting animals). Examples of Category A materials are listed in Table 1. - Category B: An infectious substance which does not pose a risk of causing permanent disability, life threatening or fatal disease to humans or animals. Most diagnostic or clinical specimens are considered Category B and are assigned UN 3373 and follow Packaging Instruction 650. - CULTURES & LABORATORY STOCKS are materials that have been amplified or propagated in order to generate high concentrations or organisms. This definition of cultures and stocks refers to items prepared for the intentional generation of pathogens and does not include cultures intended for diagnostic and clinical purposes. Cultures that meet the criteria of Category A or B infectious substances must be handled accordingly. - GENETICALLY MODIFIED ORGANISMS (GMOs) are class 9 Dangerous Goods. GMOs are defined as an organism in which genetic material has been purposely altered in a way that does not occur naturally. These materials are assigned UN 3425 and should be packed according to Packing Instruction 602. The maximum quantity in a primary receptacle must not exceed 100 ml. or 100 g. If GMOs meet the definition of Category A or B infectious substances, they must be handled accordingly. - DRY ICE is a class 9 Dangerous Good. Packages containing dry ice must always be declared as such by proper marking and labeling. A shipper’s declaration form is not required if no other dangerous goods are in the shipment. Dry ice must be packaged to permit the release of carbon dioxide gas and to prevent a build-up of pressure that could rupture the packaging. Packaging Instruction 904 must be used. A dry ice shipper’s checklist is in the warehouse. - Special packaging and arrangements must be made with the carrier for packages containing liquid nitrogen, except in the case of “Dry Shippers”. - BIOLOGICAL PRODUCTS may be considered dangerous goods and are divided into two groups. o Products manufactured and packaged in accordance with the requirements of appropriate national authorities (such as FDA). These products must be planned for use for personal health care by medical professionals or individuals. If these items are being transported for the purposes of final packaging or distribution they are not subject to dangerous goods regulations. However, Packaging Instruction 650 is recommended. o Examples of non-regulated biological products may include vaccines; licensed diagnostic kits; experimental product distributed prior to licensing; investigational new drugs; items used for prevention, treatment, or diagnosis of disease in humans or animals; and items used for development, experimental or investigational purposes. Substances which are not described above and are known or reasonably believed to contain infectious substances may meet the criteria for inclusion in Category A or Category B. If substances meet the definition of Category A or B materials, then they must be handled as an infectious substance. TABLE 1: CATEGORY A INFECTIOUS SUBSTANCES UN Number & Proper Shipping Name Organism Name UN 2814: Bacillus anthracis (cultures only) Infectious substances Brucella abortus (cultures only) affecting humans Brucella melitensis (cultures only) Brucella suis (cultures only) Burkholderia mallei - Pseudomonas mallei – Glanders (cultures only) Burkholderia pseudomallei – Pseudomonas pseudomallei (cultures only) Chlamydia psittaci - avian strains (cultures only) Clostridium botulinum (cultures only) Coccidioides immitis (cultures only) Coxiella burnetii (cultures only) Crimean-Congo hemorrhagic fever virus Dengue virus (cultures only) Eastern equine encephalitis virus (cultures only) Escherichia coli, verotoxigenic (cultures only) Ebola virus Flexal virus Francisella tularensis (cultures only) Guanarito virus Hantaan virus Hantaviruses causing hantavirus pulmonary syndrome Hendra virus Hepatitis B virus (cultures only) Herpes B virus (cultures only) Human immunodeficiency virus (cultures only) Highly pathogenic avian influenza virus (cultures only) Japanese Encephalitis virus (cultures only) Junin virus Kyasanur Forest disease virus Lassa virus Machupo virus Marburg virus Monkeypox virus Mycobacterium tuberculosis (cultures only) Nipah virus Omsk hemorrhagic fever virus Poliovirus (cultures only) Rabies virus Rickettsia prowazekii (cultures only) Rickettsia rickettsii (cultures only) Rift Valley fever virus Russian spring-summer encephalitis virus (cultures only) Sabia virus Shigella dysenteriae type 1 (cultures only) Tick-borne encephalitis virus (cultures only) Variola virus Venezuelan equine encephalitis virus West Nile virus (cultures only) Yellow fever virus (cultures only) Yersinia pestis (cultures only) UN 2900: African horse sickness virus Infectious substances African swine fever virus affecting animals only Avian paramyxovirus Type 1 - Newcastle disease virus Bluetongue virus Classical swine fever virus Foot and mouth disease virus Lumpy skin disease virus Mycoplasma mycoides - Contagious bovine pleuropneumonia Peste des petits ruminants virus Rinderpest virus Sheep-pox virus Goatpox virus Swine vesicular disease virus Vesicular stomatitis virus Required Steps for Shipping Dangerous Goods. Shippers are responsible for following the steps below when shipping dangerous goods: 1. Classify. The materials must be classified according to hazard class 1-9 (ex. infectious substances are class 6.2, GMOs are class 9). Any additional hazards present such as dry ice or flammable materials must also be identified. 2. Identify. From the IATA Dangerous Goods Manual, identify a proper shipping name, UN ID number, hazard class, packing group, cargo/passenger air limitations and special provisions. 3. Select the Proper Packaging. The IATA Dangerous Goods manual sets out which packing instructions should be used. An example of a triple-packaging system for packaging infectious, diagnostic or biological shipments is shown on page 5. 4. Pack. Follow the directions provided by the packing material manufacturer. Make sure all of the components are included. Ensure a leak proof seal. Be sure to tape the screw cap of the primary containers. (Please note that all samples should be packed within the lab environment by the customer, CME staff, do not re-pack UN2814). 5. Mark and Label. Labels identify that packages contain dangerous goods & give a broad indication of the type of material inside the box. Mislabeling or misrepresenting package contents is illegal. 6. Complete the Documentation. All shipments of dangerous goods must be accompanied by an airway bill or shipper’s declaration that describes the consignment in detail. For an infectious substance, both a shipper’s declaration and a 24-hour emergency response telephone number are required. 7. Forward Pre Notification of Shipment Arrival. Check if there are any special handling or permit requirements for the shipment. Ensure the consignee is aware of the package, its contents, temperature requirement routing and ETA. Prohibited Items and Prohibited Modes of Transportation - Certain items and substances are prohibited from transportation by Air; check the IATA DG Regulations, also dangerous goods by hand or in luggage is only permitted if explicit approval is given from the carrier. Dry ice, only if declared to the carrier and approved, may be put in carry-on or checked baggage. - IATA regulations cover any dangerous goods that are carried or transported including when boarding an airplane. Persons who violate IATA regulations and knowingly misdeclare items on a DG note are subject to fines and criminal prosecution. Labels & Packaging Infectious substances and other dangerous goods must be transported in appropriate containers. UN approved packaging materials meet stringent “test” criteria ensuring that the package can withstand leakage, shocks, temperature changes and pressure changes that can occur during transport. These UN approved packaging materials are acceptable for transport of shipments in motor vehicles, trains, boats and airplanes. Labels: If package contains infectious materials, a class 6 infectious substance label must be placed on the outer packaging. A new diamond shaped label printed with UN 3373 is required for all diagnostic or clinical specimens. If dry ice is present in the shipment, a class 9 diamond label must be on the outer packaging. When shipping over 50 ml or 50g of infectious substance, you must also either split the shipment down into the appropriate number of boxes or put a “Cargo air craft only” label on the outer package. Receiving Dangerous Goods Packages The following provisions are incorporated within the SOP for receipt of packages containing infectious substances, diagnostic specimens or GMOs that require re packing. CME staff must not open any inner packing’s, but will check for the following: Proper paperwork and labelling: - The label and accompanying documentation should be examined. Package integrity: - The package should not be leaking or appear damaged in any way. If it is, notify the PI immediately. Disinfection & clean-up materials should be available for spills. Leaking Packages: - Receiving warehouse staff should not handle a package that appears to be leaking or damaged. Receiving personnel should isolate the area around the package, and then notify the recipient and the 24 hour emergency contact. If anyone has become contaminated after handling the leaking package, they should wash the affected area for 15 minutes, and then visit the local (A&E at the Royal London Hospital, Whitechapel Road E1) for additional medical follow-up. The trained representative or if in doubt local authority (Fire brigade – dial 999) should clean up any spill and decontaminate the area according to procedures listed below. Clinical Trials and Patient Specimens A Clinical Trial is an experiment on human beings and is controlled by local government. Principle controls are the Declaration of Helsinki, which is designed to give protection to the patient, or subject; and Good Clinical Practice, which drills down into more detail and additional requires integrity of the data produced. GCP overlaps with GDP in certain aspects and it is imperative that when CME is utilised to provide logistics for clinical trials, we must be involved with the trail co-ordinator pre start up and agree the SOP’S. If storing clinical trial materials, protocols must be set up and agreed with the client prior to trial commencement as it is a requirement of GCP that monitoring of packaging, distribution and storage is approved by both regulatory and technical bodies. CitySprint Medical Express have been chosen by various labs within UK University research campuses to assist with the logistical set up and going support of Clinical Trials. Many labs have been let down by the larger integrators, who simply have not collected the patient samples, lost patient samples or in some cases have left patient samples on airport runways in direct contact with sunlight for the samples to be rendered useless. As part of our Clinical Trial Support, we work alongside the clinical trial managers or trial coordinators: - Contact the local collaborator or research nurse to understand how the samples are presently stored (frozen, chilled, and ambient). - Forward to the collaborator the correct packing material and dry-ice arrange a predetermined time when the lab will be ready to hand over the samples Make the booking to collect the samples, documenting collection time and ETA of arrival. - Provide on-site storage of samples collected in batches to be delivered to the study coordinator in one hit Storage, call-off and forwarding of randomised drug trial kits. All Clinical Trail activities must be handled (CME) by either: - Andrew Turner, 1588 - David Potter, 1589 - David Humby, 1590 Cold Chain, Distribution & Warehousing Cool Chain Solutions CitySprint is committed to maintaining, developing, controlling and ensuring the cold chain environment for clients is our number one priority. Our own internal auditors and scientific team will work closely in partnership with clients at the outset to ensure that we understand the current requirements, prepare and agree auditable SOPS; ensuring valid audits are undertaken by client staff to ensure that we jointly agree a Global Shipping solution fully tailored to the requirements of the client. Within the UK we operate our own fleet of temperature-controlled vehicles, which maintain preprescribed temperatures for each specific cargo. Within the UK we also offer a vast range of temperature controlled packing options for smaller items, ranging from Liquid Nitrogen, Dry-Ice, Wet-Ice solutions, and Chilled Gel solutions to small controlled release shipping boxes with in-built temperature monitors. For shipments overseas we have partnered with “Envirotainer” utilising their temperature controlled shipping products, which maintain the integrity of chilled or frozen samples up-to 96 hours depending on the unit utilised. The Envirotainer units are leased by CME and we handle the booking and return of each unit. Outside of the UK we also offer a vast range of temperature controlled packing options for smaller items, ranging from Liquid Nitrogen, Dry-Ice, Wet-Ice solutions, and Chilled Gel solutions to small controlled release shipping boxes with in-built temperature monitors. Envirotainer leases must be made at least 24 hours prior to requested shipping date. Lease forms are attached and must be faxed to: - Lease form - Return form - Unit specifications Best Practice Solutions for Temperature Ranges and GDP Packing materials, thermal control units, dry-ice and refrigerants are held in stock in the Medical Unit in the Warehouse. We provide 3 main thermal control units, available for either PI650 or PI602 materials. These are our validated 96 Hour validated shipper, used for overseas samples to North America, Asia, Australasia, India, Africa our 72 Hour validated shipper used for sending frozen or chilled samples within Western and Eastern Europe and our 48 Hour validated shipper for use within the UK. The 48 Hour Shipper is priced at £15.95 plus vat, the 72 Hour Shipper is priced at £20.95 plus vat, and the 96 Hour Shipper is priced at £35.95 plus vat. Also available to clients are our Liquid Nitrogen Dry-Shippers, these are normally hired out to labs for shipping samples in Liquid Nitrogen. CitySprint Medical Express also stock a full range of UN approved packing materials for almost any potential combination of ambient or temperature controlled shipment, for all packing groups and classes. For further information and bespoke request please contact David Potter on 1589. In addition we also utilise Envirotainer units for highly sensitive and high valuable items. We have a working relationship with Envirotainer and their LHR hub is1 minute from CME’s LHR offices. When agreeing an SOP with the client it is imperative that the destination is qualified to ensure that the appropriate packing and cool chain environment is developed. Temperature validation can be provided utilising a TempTale. Temp Tales are held in stock in the Medical Room in the warehouse and are available in single or multi use units. The multi use units are reserved for on going clinical trials. Under no circumstances must a Temp Tale be used without agreeing the Temperature range with the client and validating the cold chain environment and routing. Temp Tales must be ordered through Andy Turner, 1588 or David Potter 1589 only! CME do not dictate the Temperature Range when shipping samples this must be done by the client. Chain of Custody CME as part of the SOP for shipping Clinical or Pharmaceutical goods will agree an effective Chain of Custody with each clinical trial or Pharmaceutical client. Many precautions are taking in a typical research lab to ensure the integrity of biological specimens, clinical trial investigative drugs and vaccines. Temperature and storage are tightly controlled by our clients and as such CME must demonstrate similar controls within it’s SOP’s. To ensure that CME adheres to the strict protocols adopted by our customer there are various forms and manifest which are used when handling Pharmaceutical and clinical commodity’s through our cold chain. 1. The cold chain begins at the client site and is handed over to CitySprint once the collection is made from the client site, either in a refrigerated vehicle or a temperate vehicle. 2. The shipment is manifested into the local warehouse where it is either on forwarded to the destination country via a direct and specific routing or it is stored in a cold room, fridge or freezer (if prescribed) for delivery the following day. 3. Electronic audit trail and hard coded collection and delivery information is captured and can be viewed on line at www.citysprint.co.uk. CitySprint are able to provide On Line Hard Copy Proof of Deliveries, instantly upon delivery. 4. Each of the drivers is tracked via satellite tracking and their exact whereabouts can be viewed on line at www.citysprint.co.uk 5. Once in transit the temperature of certain items including vaccines, blood products and plasma and clinical trial material should be monitored at regular pre determined internal by utilising a Temp Tale unit. 6. For international shipment the Airline Check form should be completed where possible, this is used in conjunction with THE Temp Tale readouts. In most if not all cases this information is collected by the clinical trail company and not by CME. 7. Final delivery POD is captured, and where possible the Temp Tale unit is sent back to the client for analysis. 8. Job is closed off and temperature validation is documented and filed by client. - A custody manifest is enclosed over. - A full description of our tracking facilities is enclosed over Custody Manifests Booking and Tracking Capabilities Our clients would be able to book our services, via the telephone and On-Line, via our On-Line Booking Facility. Outlined below is a brief explanation of this process. Telephone Booking: Each booking would be made directly on to our CityTrak system. This involves the simple filling in of a certain number of fields on one screen (see screen example overleaf). Once this process is completed the job is confirmed and then electronically transferred to the relevant circuit for allocation to a courier. There are time saving features that can be used in this process including storage of regularly used collection and delivery addresses which can be accessed with a single key stroke. If the customer had any regular jobs/runs that occur at the same time each day, CitySprint would be able to upload the job onto our CityTrak platform and it would automatically appear on the controller’s screen for allocation. This means that the customer would not need to continually contact our call centre to book the same job each day. Security features can also be used in this process including: Authorised user lists Verification of cost centres and references Delivery Procedure On-Line Booking On-Line Booking is a Java front end to our CityTrak IT Operating Platform and feeds information directly into CityTrak. CitySprint’s On-Line Booking for SameDay Courier jobs is an extremely robust system, over 15,500 jobs are booked monthly and it has been in operation for more than three years. Our clients would access this system through our corporate web site, www.citysprint.co.uk and log in using their account number and a unique password. After logging in, clients’ staff would be transferred to the booking screen. Within this screen the Our clients personnel would complete a series of fields with the relevant details of their job, some of these fields are compulsory and the system will not let the booking progress until these fields are completed, an error message is displayed. The booking screen also has an address book facility. Our clients’ staff would be able to save frequently used addresses; therefore, instead of continually typing in the same addresses, the person booking can just scroll through and select the appropriate address. Additionally, CitySprint can modify the department and reference fields to any preference, such as purchase order number or departmental code. The compulsory fields are highlighted with blue writing. An example of a booking screen is displayed below. NextDay Courier and International Courier jobs can also be booked On-Line via our On-Line Booking system. Through this password protected system, customers are able to do the following: Access an On-Line quotation facility for specific services Request a collection Consignment information can be entered and a plain airway bill produced for the shipment An end of day manifest can be produced to allow drivers to check and sign for all material collected Track their consignment Invoices for prior shipments can be accessed and printed. An example of a CityTrak International screen is shown below. All shipments processed by CityTrak International travel on a bar-coded Airway bill. These airway bills are scanned at each leg of the journey from collection to final delivery and allow for real time tracking to be provided On-Line at www.citysprint.co.uk Tracking Capabilities Monitoring: From the time that a booking is made for the despatch of a consignment to the time that the consignment is delivered, CityTrak maintains a full audit trail which is recorded automatically. The points at which details are recorded are: At booking At allocation to courier At collection At delivery At each point details are recorded of The time The operator name The operation carried out This information is collected by our mobile data units, CityTrakkers, which are carried by our couriers. Details of the pick up are relayed directly to the unit by our CityTrak system. The courier confirms that they have received the details and also confirms that they have picked up the consignment. On delivery the recipient signs across the CityTrakker and the courier enters the name of the signatory. This information along with the time the signature is entered and is transmitted via the GPRS network back to our CityTrak operating platform this information is also viewable over the internet, via our On-Line Booking facility. Through our On-Line Booking system, the clients can also track the progress of their package, this is enabled through the job reports and tracking page. Clients can track the progress of the package through using either the job reference number given or the date the job was completed. After selecting the job the journey is then displayed and at the bottom of the screen is a message box detailing the latest information on the job, for example confirmation of booking and delivery time. Additionally within this system, clients would also able to view the electronic signature of the recipient. This information can be printed off and kept for future reference, an example of this proof of delivery is shown overleaf. Additionally, through the job reports and tracking page, the Our clients could access management reports detailing the amount of jobs booked and the journey details, which can be either downloaded in a CSV file or downloaded into a PDF format, which can be printed out. This information can be used to analyse usage of the services. A screenshot of this screen is displayed below. CitySprint have also implemented a single job search facility, QuikTrak. QuikTrak is also available via our corporate web site, but unlike On-Line Booking customers do not need to input a password with an account number just input the account number and the unique job reference number relating to the job, which is given at booking. The difference between this facility and our On-Line Booking facility is that this system is designed for the tracking of a single job, whereas On-Line Booking enables customers to search for more than one job undertaken within a certain period and a report can also be downloaded detailing the number of jobs booked. International Tracking and Tracing Each shipment will be scanned at each leg of the journey to ensure that tracking information is up to date. All tracking information is available on-line. Proof of Delivery will be available On-Line: Mobile Data and GPS Tracking CitySprint have equipped our national fleet with CityTrakker units. CityTrakkers are XDA mobile data units through which our control staff allocate jobs to our couriers. Once the job has been allocated the driver then confirms that they have received the details and also confirms that they have picked up the consignment. On delivery the recipient signs across the CityTrakker and the courier enters the name of the signatory. This information along with the time the signature is entered and is transmitted via the GPRS network back to our CityTrak operating platform and is also viewable over the internet, via our On-Line Booking facility. The CityTrakker units also have an industry first fail-over system through a GRPS and GSM facility. The CityTrakker can also double as a mobile phone if further communication is needed. All CitySprint couriers will have a GPS unit connected to their CityTrakker XDAs. CitySprint controllers are able to monitor the exact location of all couriers. Once a consignment has been allocated to a courier, customers would be able to view the actual progress of the courier on line, in terms of pinpointing the courier’s position on a map, until the delivery has been made and the POD is available also on line in real time. GPS mapping provides CitySprint Operations staff with full visibility of all couriers from any location. Customers can view the location of a courier allocated to their consignment from time of allocation to time of delivery. Temperature Control/Validation on site CME has one 10’ x 6’ walk in cold room, which is set for a temperature range of 2 – 8˚. This is used for the following clinical trials: Cold Room Validation is implemented on regular basis by Sensitech. When implementing clinical trials that require temperature validation this must be written in to the specific GDP/SOP for that trial and original validation reports must be sent directly to the client. - - - Police Airwave Monitoring Study o Sponsor: Home Office o Trial Coordinator: Imperial College Ministry of Defence o Sponsor: MOD o Trial Coordinator: DTMA compulsory drug testing (LGC Labs) Ad hoc sample storage o MRC o Imperial College o Baxter o MRD Leukaemia Trial o St Thomas’s Hospital o Bart’s and The London Hospital All samples for on-going trials must be pre-booked and all ad-hoc samples must be manifested in and out. See manifest form on side of unit. The units are leased from Ice Cool Trailers, 24 hour emergency response is available from 01653 250 950 or 07850 226839, account manager is Miles Atherton. Agreed SLA for call out engineer is 120 minutes from call. There is 1 Frigidaire domestic freezer and 1 Frigidaire fridge located in the medical unit in the warehouse. Temperature Validation in transit CME has partnered with Sensitech to ensure accurate temperature monitoring whilst in transit. Sensitech provides single or multi use Temp tale units that monitor a range of temperatures from temperate and chilled units to units that are placed within dry-ice shippers. All Temp Tales units must be ordered through Andy Turner, 1588 or David Potter 1589. CME’s account manager is: Simon Hartley Regional Sales Manager Sensitech UK Limited 167 - 169 Kensington High Street London W8 6SH Tel: +44 (0) 207 795 4420 Fax +44 (0) 20 7937 0661 Mobile +44 (0) 791 757 7375 The following pages relate to the set up and SOP’s for each of the Temp Tale units. TempTale 4 Instruction Sheet A TempTale 4 temperature-monitoring device is included in this shipment. Refer to the ‘Receipt of Study Vaccines at the Study Site’ Procedure in your Study Specific Manual for detailed instruction. Before stopping the TempTale 4 monitor: inspect the digital display to ensure that there is a sun icon in the upper left-hand corner. The sun icon ( ) is visible when the TempTale is recording. Switch the TempTale 4 monitor off: Press the red “Stop” button on the TempTale monitor and hold down for 3 seconds, to stop temperature measurement. The sun icon ( ) will disappear when the TempTale is stopped. A “Stop” icon ( ) appears when the TempTale is stopped. If a bell icon ( ) is displayed before / after stopping the TempTale: Vaccine must be quarantined! To obtain the temperature readings for the TempTale Receipt Form: Press the “Start” button to obtain brief readings. The readings are given in the following order: • Mean temperature (C) • Highest temperature (C) • Total time above high temperature limit (minutes) • Lowest temperature (C) • Total time below low temperature limit (minutes) S.O.P. s - Using TempTale4 2K Temperature Monitors: I. Downloading a TempTale4 Monitor: Once you have removed a TT4 monitor from a fridge, press the Stop button for 3 seconds. This will ensure that the TT4 was stop. A small “Stop Sign” shaped icon should appear on the top right corner of the LCD indicating the monitor has been stopped. 1) From the Start-up Assistant dialog, or select Monitor; Download TempTale Monitor or click on the Download a TempTale4 monitor icon. 2) At the Download Monitor dialog connect the TempTale4 to the Interface Plus by laying the TempTale4 facedown in the bracket of the Interface Plus, so the LED lights are aligned. Than click OK to continue. 3) At the prompt disconnect the TempTale4 monitor and click OK. In the case of Multi-Use monitors that are still actively recording data will display “Would you like to stop this multi-use monitor now?” 4) Click Yes to transfer the data stored and stop recording additional data. Click No to transfer the data stored and continue recording data when the TempTale4 monitor is disconnected from the Interface Plus. II. Accessing the Data: To access the Graph, Summary or Tabular views, download a TempTale4 monitor or open a stored file. Clicking in the tabs, located at the bottom of the screen, will display the Summary, Configuration, Tabular or Graph views for that file. 1) Summary View: This view displays the start and stop time, minimum temperatures recorded, average, standard deviation, time above and below the programmed range, degrees minutes above and below the programmed range and the number of events above and below the programmed range as well as comments stored with the file. TempTale4 & Software Features and Benefits Feature LCD displays key decisionmaking information Only monitor available that displays cumulative time out of range Software allows multiple files to appear on one display Benefit No need to download TempTale to view key data; can download at convenience for further analysis and documentation. Assures customers of state-of-the-art technology that competitors cannot offer. Up to 15 files can appear at once as multiple graphs overlaying and multiple tabular reports, which will be useful when doing any validation/qualification studies Data cannot be overwritten The data recorded on the unit will not be overwritten. All records remain on the data logger even after it is downloaded several times. Data cannot be altered After downloading the data cannot be altered Certificates of Calibration Certificates of Calibration are provided with all provided with both Single-Use units, which are traceable to NIST standards. and Multiple-Use monitors free You do not need to calibrate the units in-house of charge or have them sent to a 3rd party calibration company, which would cost as much as a new monitor. Customized Alarm Triggers Alarm is very useful in making accept/reject Cumulative time above or decisions. below No need to download monitor to make Single event above or below decision. Degree-minutes above or below Annual Trade-in for Multiple All Multiple-Use monitors are traded-in use monitors annually. New units along with certificates of validation will be provided for less than half of the initial unit price. Using a TempTale 4 Monitor Starting the TempTale 4 Monitor 1. Hold down the start button (the green button on the monitor) until you see the sunshine icon (picture) on the upper left corner of the LCD that confirms that the monitor has started. When the monitor is activated, the sunshine icon will stay on until the monitor has been stopped. The monitor will begin recording data after the startup delay is completed. Refer to the figure below: TempTale 4 Monitor Note: TTM software uses only temperature data to generate graphs, tables, and summary data. - PROGRAMMING OPTIONS Catalogue Number: Alarm Bell on LCD: Certifcates: Start-Up Delay: Display Current Temp: Choose one a Recording Period: b Measurement Interval High Alarm Delay Low Alarm Delay Launch Options: LCD Panel Display Start Key Options: Stop Key Options: Temperature Range: Monitor Type: Single Use Y/N (mins) Y/N Days/Hours International Certificate of Validation 30 mins Yes 5 mins C (mins) C (mins) C or F 8 0 2 0 Manual, 30 min delay C 3 second delay 3 second delay 2-8C Configuring a TempTale 4 Monitor 1. Open TTM software and select Start Up Assistant; Configure a TempTale Monitor. The ‘Monitor Configuration’ dialog box will open. 2. Connect the monitor facedown on the Interface Plus and select OK. 3. If the Monitor is Single Use and has been used you will not be allowed to re-configure the unit. 4. Otherwise edit the configuration data in both fields General Configuration and LCD Alarm accessible by the index tabs on top of the Monitor Configuration window. 5. Confirm new settings are correct as only one configuration change on Single Use loggers is permitted. Then select OK. New data will be stored. When completed, remove logger and select OK. Screen on TT4 should be blank, this is now ready for use. Setting Alarm Criteria (TT4 monitors only) Click on the index at the top of the screen for the LCD Alarm view. This menu is used to set the alarm criteria for TempTale 4 monitors. LCD Alarm View: Description of fields - Alarm Criteria: If enabled, the alarm will utilize the user defined alarm settings. - Temperature Limit: This is the temperature used for the low limit or high limit alarm. When setting the low alarm, the alarm is triggered if it drops below this temperature for a set period, defined by the Alarm Type and “Threshold” fields. When the high alarm, the alarm is triggered if it goes above this temperature for a set period, defined by the Alarm Type and “Threshold “ fields. The temperature range is from –4 to +158 degrees Fahrenheit or –20 to +70 degrees Celsius, in whole degrees only. - Alarm Type: The user must select from a predefined list of two choices for this field: “Time-Single Event” or “Time Cumulative”. Each of the four choices is described below in more detail. - Time-Single Event: Alarm triggers if temperature is above/below ideal range for a single event of the defined threshold. - Time- Cumulative: Alarm triggers if temperature is above/below ideal range for the cumulative time of the defined threshold. Threshold: The range allowed is from 0 to 999 minutes. Documentation and Local Regulatory Paperwork The following documents are samples of the paperwork that is prepared on behalf of our customers, and includes: - Commercial Invoices - Dangerous Goods Shippers Declaration - Import Permit Applications Qualified and Trained Personnel CitySprint representatives are on hand 24/7 to provide advice to clients on the appropriate method of transportation for hazardous goods, non-hazardous patient samples, medical instruments and biological samples. The representatives would facilitate the collection of these consignments as well and be on hand to answer any queries that the client’s personnel may have regarding proper packing instructions, Dangerous Goods paperwork and the requirements for obtaining local country import/export licences. The IATA trained DG representatives will classify the samples and check the UN number(s) to ensure they are appropriately packaged as per the correct IATA Packing Instruction: For example: UN3373 Diagnostic Specimens = IATA PI650 UN2814 Infectious Substance affecting Humans = IATA PI602 UN1230 Methanol = IATA Y305/305/307 Or “Exempt Human Specimen” same packing instruction as PI650 The IATA/ADR trained CitySprint representatives available are: Andrew Turner: 24 hour emergency contact number – 07989 857 816 David Potter: 24 hour emergency contact number – 07989 544 424 Paul Guerrier 24 hour emergency contact number – 07957 545 628 The CitySprint DGSA is: Tracy Fletcher: 24 hour emergency contact number – 07930 481 009 CitySprint’s Competent Regulatory Contacts: DEFRA: Ms Kirsty Glover - 020 7904 6790 DEFRA: Mr Paul Manser - 020 7904 6187 DOT: Andrea Pearson - 020 7944 2763 Health Canada: Ms Stacey Mantha, Head, Biosafety Services – 001 613 957 1779 IATA: Mr Dave Brennan - 001 800 71 66 32 60 USDA: Dr Morris - 001 301 734 3277 USDA: CMDR Bill Burkhardt – 001 251- 690-3361 CDC: Mr Mathew Jennings - 001 404 498 2260 DGSA Our Dangerous Goods Safety Adviser service will offer advice on the following. - Monitoring compliance with the rules governing the transport of dangerous goods. - Advice on the transport of dangerous goods. - Ensuring that an annual report is prepared on the activities concerning the transport of dangerous goods. - Monitoring the procedures for compliance with the rules governing the identification of dangerous goods being transported. - Monitoring the procedures for checking the equipment used in connection with the transport of dangerous goods. - Monitoring the proper training of employees and the maintenance of records of such training. - Monitoring the implementation of appropriate measures to avoid the recurrence of accidents, incidents or serious infringements. - Monitoring the account taken of the legal prescriptions and special requirements associated with the transport of dangerous goods in the choice and use of subcontractors or third parties. - Verification that employees involved in the transport of dangerous goods have detailed operational procedures and instructions. - Introduction of measures to increase awareness of the risks inherent in the transport of dangerous goods. - Implementation of verification procedures to ensure the presence, on board the means of transport, of the documents and safety equipment which must be accompanied during transport and the compliance of such documents and equipment with health and safety regulations. - The implementation of verification procedures to ensure compliance with legislation governing loading and unloading dangerous goods. MAJAX and Disaster Recovery Purpose: This document is intended to establish working guidelines in conjunction with our customers for the effective transition and business continuity in the event of a Major Incident or Disaster. Emergency Response Procedures For detailed emergency response guidelines CitySprint Medical Express follow relevant HSE/COSHH guidelines and as an aide-memoir CitySprint Medical Express prescribe to a US Government publication, the Emergency Response Guidebook, which can be viewed at http://hazmat.dot.gov/erg2000/erg2000.pdf. This document lists in detail emergency response guidelines for Dangerous Goods. Disaster Recovery: CitySprint have invested heavily to ensure business continuity. All areas of our systems have been analysed and appropriate disaster recovery procedures laid down. The key areas covered are as follows:- Building Damage - London In the case where our Scrutton Street offices become inoperable we have a contract with NDR that allows us to occupy floor space at any of their recovery centres. They have 2 separate sites in London and 1 in the Midlands. At this centre NDR have prepared a range of facilities for CitySprint. These facilities include o 50 PC desk positions o 50 Telephones o 2 MB Internet connection o Telephony connections to all major telecom suppliers o Full range of servers for CitySprint to reload their key systems on o All numbers can be quickly diverted by our Telecom suppliers BT and MCI Building Damage – Outside of London All CitySprint offices operate a ‘buddy’ system. Should a building become inoperable the telephone numbers are diverted to the ‘buddy’ office from where the customer can be cared for until the office is re-instated. Telecoms Failures CitySprint have a 24 hour, 7 day a week, 52 week a year support contract with BT and MCI. Extensive use of non-geographic numbers allows CitySprint to divert telephone numbers to any other UK location within 24 hours. Standard geographic numbers are diverted by request with our telecom suppliers All key data communication lines are backed up by means of either 2 MB broadband connections or ISDN-2 dial up connections, and in some cases both. Power Supply Failure Scrutton Street has a 3 tiered approach to power failure. All servers have an individual UPS connected to them. The entire building is also protected by a single large UPS that will keep all services running whilst our on site generator starts. The generator is an F G Wilson P380 KVA, it is covered by 24 hour, 365 days a year call out insurance, and if there are any issues our maintenance provider is on call to resolve the issue. Our maintenance provider is Bills Control Ltd, who is based in Scrutton Street, just a few doors down from our own premises. So if we needed to call the engineers out, they have a quick response time. The generator has an engine capacity of 14 BMW 7 series cars, and has the capacity to fulfil the building’s needs five times over. System Hardware Failure CitySprint have contracted CCE engineering to maintain all our hardware on a national basis. All hardware is covered by next day fix, on site anywhere in the UK mainland, thereby limiting the impact of a breakdown. All servers have a 4 hour fix time associated with them. Some key systems can never be unavailable and consequently we operate complete hardware duplication. The systems duplicated are: o CityTrak Server o Web and On-Line Booking Server o Mobile Data Gateways Data Loss CitySprint are highly conscious of the importance of data backups. All servers and user data are backed up every night with an incremental backup. A full system wide backup is taken every weekend. In order to ensure that the latest backups are not held on-site CitySprint have contracted Recall to collect the latest backup tapes every day and return the previous week’s daily backup for that day. The rota ensures that at anytime we keep a full copy of all our data off-site. Daily backups are not always sufficient and on our CityTrak server we take a 2 hourly backup and a full daily backup. Later in 2006 CitySprint will be upgrading CityTrak to a transactional backup so every single transaction made is immediately copied to our duplicate failover server. This mirroring ensures that CityTrak will never be off-line or lose data. Similarly our On-Line system also has duplication that allows a seamless failover to the duplicate server. MHRA/DOT CME follow closely the guidelines prescribed by the MHRA in the UK and by the DOT in the US. Particular attention is paid to the DOT publication 49 CFR part 171 when shipping to the US. US customs entry requirements are often complex and involve clearance from: - US Customs - CDC - USDA - FDA The USDA and CDC websites contain informative information and list the requirements for shipping into the US. - www.cdc.gov - www.usda.gov It is imperative that all consignments to the US are properly prepared prior to forwarding. You must not forward any medical samples to any country, especially the US without checking if an import permit is required. Check the relevant Government website. When importing back to the UK checks with DEFRA are required for Animal Samples or Human Samples in any form of culture medium derived from animals. - www.defra.gov.uk