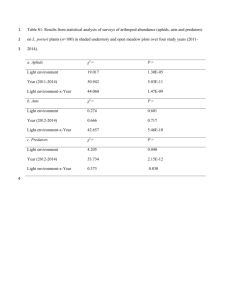
Effects of habitat type on ground-dwelling ant assemblage in a
advertisement

LAURENCE THEUNIS Thèse de doctorat présentée en vue de l’obtention du grade de docteur en Sciences Biologiques LEAF LITTER ANT ASSEMBLAGE IN A NATURAL FRAGMENTED DRY FOREST IN THE ARGENTINIAN CHACO PROMOTEURS: PROF. J.M. PASTEELS – PROF. Y. ROISIN (ULB) DR. M. LEPONCE (IRSNB) TABLE DES MATIERES INTRODUCTION GENERALE 1. Caractérisation d’un assemblage 2. Le choix de l’échelle spatiale 3. Le morcellement naturel de la forêt 4. Système d’étude 4.1 Historique de la genèse du parc national Rio Pilcomayo 4.2 Feux et inondations 4.3 Climatologie 4.4 Végétation et faune principale 5. Fourmis des litières 6. Matériel et méthodes 6.1 Protocole d’échantillonnage 6.2 Méthodes de récolte des fourmis des litières 6.3 Tri des échantillons et identification de espèces 6.4 Statistiques CHAPITRE I Leponce, M., L. Theunis, J.H.C. Delabie and Y. Roisin, 2004. Scale dependence of diversity measures in a leaf-litter ant assemblage. Ecography, 27: 253-267. Theunis, L.; Gilbert, M.; Roisin, Y. & Leponce, M. (Accepted in Insectes Sociaux) Spatial structure of litter-dwelling ant distribution in a subtropical dry forest. Chapitre II Laurence Theunis, Yves Roisin, Jacques H.C. Delabie and Maurice Leponce. Effects of habitat type on ground-dwelling ant assemblage in a fragmented forest of the humid Chaco. Laurence Theunis, Yves Roisin and Maurice Leponce. Effects of the presence of terrestrial bromeliads on leaf litter ants in a Chacoan forest. CHAPITRE III Laurence Theunis, Yves Roisin and Maurice Leponce. Effects of natural forest fragmentation on the structure of a leaf litter ant assemblage in the humid Chaco. DISCUSSION GÉNÉRALE CHAPITRE I Spatial structure of litter-dwelling ant distribution in a subtropical dry forest L. Theunis 1, 2, M. Gilbert 3, Y. Roisin 2 and M. Leponce 1 (Accepted in Insectes Sociaux) 1 Section of Conservation Biology, Royal Belgian Institute of Natural Sciences, Rue Vautier 29, B-1000 Brussels, Belgium, e-mail: Laurence.Theunis@naturalsciences.be 2 Behavioral and Evolutionary Ecology, CP 160/12, Université Libre de Bruxelles, Avenue F.D. Roosevelt 50, B-1050 Brussels, Belgium. 3 Biological Control and Spatial Ecology, CP 160/12, Université Libre de Bruxelles, Avenue F.D. Roosevelt 50, B-1050 Brussels, Belgium. Laurence Theunis Phone +32 2 627.43.64 Fax +32 2 649.48.25 E-mail: Laurence.Theunis@naturalsciences.be RUNNING HEAD: Spatial structure of ant distribution Keywords: Spatial pattern, ant distribution, geostatistics, Chaco. Summary. Understanding the spatial patterns of species distribution is essential to characterize the structure of communities, to optimize species inventories and to evaluate the impact of biotic and abiotic variables. Here we describe the spatial structure of the distribution of leaf litter ant species, and of biotic factors that could explain it, in a subtropical semi-deciduous forest of the Argentinian Chaco, characterized by a dense understorey of shrubs and terrestrial bromeliads. Environmental variables (leaf litter quantity and ground bromeliad density) were measured and ants were collected in 1m² quadrats distributed along two 200m transects at intervals of 1.25m. Overall 87 species were collected. Sixteen positive associations and a single negative association were observed between the 11 most frequent species taken pair-wise. Our results suggest that the spatial distribution of leaf litter ants was determined at two different scales. At a small scale (period below 10m) a periodic spatial structure, likely due to intraspecific competition, produced a succession of peaks of abundance separated by gaps. At a larger scale (period around 50m), periodically distributed environmental factors induced aggregates of colonies of species responding positively to these factors. A high quantity of leaf litter and, to a lesser extent, a high density of ground bromeliads promoted a high density and a high species richness of ants. Numerically dominant ants being generally positively associated, interspecific competition was apparently weak. All ant species whose abundance was correlated with an environmental factor were not completely spatially structured by it. This suggests that some other factors, such as intraspecific competition, may have counter-effects. Introduction Understanding the spatial patterns of species distribution is essential to characterize the structure of communities, to optimize species inventories (Leponce et al., 2004) and to evaluate the impact of biotic and abiotic variables. Little is known about the fine spatial scaling of the majority of species assemblages including leaf litter ants. Tropical ant assemblages show a high species richness and a patchy distribution of colonies (Wilson, 1958; Levings and Franks, 1982; Levings, 1983; Benson and Brandão, 1987; Kaspari, 1996a; Vasconcelos and Delabie, 2000) which depends on biotic and abiotic constraints. Leaf litter ants are not territorial and a considerable amount of evidence suggests that favourable resource availability, rather than competition, is a major force structuring tropical leaf litter ant assemblages (Franks, 1982; Byrne, 1994; Kaspari, 1996a,b; Soares and Schoereder, 2001) involving overlapping foraging areas (Jackson, 1984; Byrne, 1994). For ground-dwelling ants, causes of patchiness include predation by swarm-raiding army ants (Franks and Bossert, 1983; Kaspari, 1996b; Hirosawa et al., 2000), moisture content preferences (Levings, 1983; Levings and Windsor, 1984; Kaspari, 1996a), temperature preferences (Bestelmeyer, 2000), topography (Vasconcelos et al., 2003), nest-site and food availability (Herbers, 1989; Byrne, 1994; Kaspari, 1996b; Kaspari and Majer, 2000), leaf litter quantity and quality (Vasconcelos, 1990; Höfer et al., 1996; Kaspari, 1996a; Carvalho and Vasconcelos, 1999) and both vegetation structure and composition (Wilson, 1958; Gadagkar et al., 1993; Feener and Schupp, 1998; Moutinho, 1998; Retana and Cerdà, 2000; Bestelmeyer and Wiens, 2001). In a previous study carried out at a high resolution and based on a nearly exhaustive sampling of a strip of 200m² in a subtropical semi-deciduous forest of the Argentinean Chaco, we demonstrated the highly heterogeneous distribution of leaf litter ant species and evaluated its consequences on diversity estimates (Leponce et al., 2004). The present study aimed at extending this work by the spatial analysis of the ant species distribution and of the biotic factors that could explain it. To achieve this objective, we measured conspicuous environmental variables likely to affect ant distribution and measured the nature of interactions between numerically dominant ants. Methods Study site The study site was located in Río Pilcomayo National Park, northern Argentina, in the wet Chaco region (25°04’06’’ S, 58°05’36’’ W). The habitat, called "monte fuerte" is a subtropical mesoxerophile oligarchic forest (Pujalte et al., 1995; habitat unit PHYSIS 48.2412 of Devillers and Devillers-Terschuren, 1996) dominated by Schinopsis balansae Engl., Astronium balansae Engl. and Aspidosperma quebracho-blanco Schlecht. and by a ground strata of bromeliads (Aechmea distichantha Lemaire and Pseudananas sagenarius (Arruda) Camargo) (Pujalte et al., 1995). Sampling design Ant sampling protocol Two 200m-long transects (A and B) located 400m apart were sampled between July 23 and August 8, 2000 in a 16ha forest fragment. Each transect consisted of 160 quadrats of 1m² separated by 1.25m intervals (transect A is extensively described in Leponce et al., 2004). At each sampling point, the leaf litter found inside the 1m² quadrat was collected, sifted and put in a cotton bag. The sifted material was brought back to field laboratory and its fauna was extracted with a mini-Winkler apparatus (Fisher, 1998) for 24 hours. Temperature, recorded every 10 minutes, ranged between 3.6°C (at night) and 27.6°C with an average of 14.1 ± 4.1°C during the sampling session of transect A and between 10.6 and 30.2°C (18.5 ± 4.2°C) during sampling of transect B. Average temperatures were lower (14.1°C) during the sampling of transect A than during the sampling of transect B (18.5°C) (t-test, p< 0.001). The weather was dry during the 17 days sampling campaign (only three short and light rains occurred). Environmental measures In order to interpret the pattern of species distribution, we measured three conspicuous environmental variables at each 1m² quadrat: (1) the sifted litter weight (which integrates factors such as food, nest, temperature and moisture availability) (Levings, 1983) (2) the density of ground bromeliads (omnipresent in the habitat and affecting ant species density and composition (unpublished results)), (3) canopy openness (influencing the temperature and dryness at ground level). The percentage of canopy openness was estimated from hemispherical photographs, shot 1.5m above ground level and quantified with the Gap Light Analyzer 2.0 program (Frazer et al., 1999). Data analysis All ants were determined to species or morphospecies level. In order to assess the impact of environmental variations on ant density and species composition, we pooled the data from the two transects. By contrast, the two transects were considered separately for the analysis of spatial structure. Numerically dominant ant species were defined as species found in at least 10% of the samples, and will be hereafter referred as “frequent species”. Faunal similarity between transect A and B was estimated using Jaccard’s index (Jaccard, 1912; Wilson and Schmida, 1984) calculated as follows: Sj = c (where a = total number of species in sample A, b = total number of species in abc sample B, c = number of common species to samples A and B). Species associations and correlations between environmental factors and ant abundance were evaluated on the log10 (n+1)-transformed abundance in order to limit the weight of samples collected around nests, trails and exploited resources. Standard parametric tests of significance could not be used here because of spatial autocorrelation (SA), which represents a bias to the assumption of independence among samples (Lennon, 2000; Legendre et al., 2002). Using simulation data, Legendre et al. (2002) showed that Dutilleul's modified t-test (Dutilleul, 1993) constitutes an efficient method to account for SA in estimating the significance of the correlation between two autocorrelated variables, and this method was used here to test the significance of all bivariate correlations. We re-adjusted the p-values for statistical acceptance with the Holm procedure (1979) (Legendre and Legendre, 1998) because the probability of a type I error becomes larger than the nominal value of α when several tests of significance are carried out simultaneously (i.e. in a correlation matrix). Spatial analysis: autocorrelation and periodicity Two methods were used to explore spatial patterns in environmental factors and ant species distributions. First, spatial correlograms were used to quantify the level of spatial dependence, i.e. the tendency of points close together to have more similar values than points farther apart. Spatial correlograms plot the values of the spatial correlations between observations separated by increasing distance classes, and allow describing the extent (distance over which no SA is measured), and intensity (when autocorrelation is strong, points separated by close distances have strongly correlated values) of SA (Rossi et al., 1992; Liebhold et al., 1993, Legendre et al., 2002; Liebhold and Gurevitch, 2002; Perry et al., 2002). Correlogram values range from -1 to +1 (Rho(h)) and can be interpreted as indicating negative or positive correlations in the same way as simple correlation coefficients. Second, periodograms were used to quantify the presence of periodic patterns in the transect data. Periodograms resulted from a Fourier-transformation decomposing the observed transect data into a sum of periodic terms, and plotting the intensity (as measured by the amplitude) as a function of the period of each term (Shumway, 1988; Legendre and Legendre, 1998). We ranked the level of periodicity in our transect data according to three arbitrary classes of amplitude: strong periodicity (highest peak > 6), intermediate (highest peak is < 6 and > 1) and low (highest peak < 1). Correlograms and periodograms were calculated using Statistica 6.0 software (StatSoft Inc, 2004). Studies of spatial patterns along transects (representing a single dimension) allow obtaining fine SA coefficients and periodogram values (Legendre and Fortin, 1989). Structuring effects We aimed at exploring whether spatial periodicity observed in species distribution could be attributed to one or several environmental covariates. The periodograms and correlograms of the residuals from the linear regression between species abundance and a microhabitat factor were therefore estimated, and compared to those of the species abundance data. A strong structuring effect of a microhabitat factor on the species distribution would result in a substantial reduction in the amplitude of the highest peak in the periodogram of regression residuals once the variability related to microhabitat factor has been removed. This reduction was quantified and used as an estimate of the spatial structuring effect of the environmental variable: decreases over 50%, between 50% and 20%, and lower than 20% were considered as strong, intermediate or low structuring effects, respectively. A similar approach was used to explore the relationship between the environmental factors. Results Eighty-seven species corresponding to 1880 occurrences and 24114 individuals were found in the 320 quadrats of the two transects (species list in Appendix 1). Both transect had 11 frequent species in common which occupied similar ranks of occurrence (Spearman rank order correlation coefficient, r= 0.691, p< 0.05). Sixteen positive and a single negative associations were observed between these 11 frequent species (species found in at least 10% of the samples) (Table 1). Relationships between ant density and environmental factors Median leaf litter weight was 357 g (quartiles: 212-524), bromeliad density 2 plants/m² (0-4) and canopy openness 18.4 % (16.9-19.9) (N=320) along transects A and B. Because values of canopy openness varied very little (variation of ±5%) (Fig. 1), we did not undertake further investigations of its effects on ant distribution. Litter quantity varied considerably, up to 25 fold between contiguous quadrats. Species density (number of species/m²) was positively correlated (Pearson’s correlation) with leaf litter weight (N= 320, r=0.71, p< 0.05) and with bromeliad density (N= 320, r= 0.31, p<0.05). Leaf litter weight was also positively correlated with bromeliad density (N= 320, r=0.27, p< 0.05). Quadrats devoid of bromeliads (N= 85 out of 320) had significantly less leaf litter (Mann-Whitney rank sum test U= 5091, p<0.001), a lower ant species density (MW rank sum test: U= 5150, p<0.001) and a lower species richness (47 vs. 55 species for 314 occurrences) than quadrats with bromeliads (N= 235). Relationships between ant species composition and environmental factors The abundance of eight frequent species was positively correlated with leaf litter weight and that of two species with bromeliad density (Table 2). Solenopsis sp.01 and Paratrechina sp.02 were positively correlated with both leaf litter weight and bromeliad density. Crematogaster sp.02, Solenopsis sp.17 and Pheidole flavens did not show any significative correlation with either litter weight or bromeliad density. Spatial pattern of environmental factors and ant distribution The spatial distribution of the environmental factors and of the 4 most frequent species (present in at least 1/3 of samples) along transect A is presented in Fig. 1. All variables except canopy openness varied significantly along the transect, with a succession of peaks and gaps. Similar results were obtained for transect B, except around a depressed zone of 15m long that was temporarily flooded and devoid of both bromeliads and leaf-litter. Leaf litter weight and bromeliad density showed a strong spatial structure in their distribution along transects A and B (Fig. 2). Leaf litter quantity correlogram indicated evidence of a periodic spatial distribution along both transects (Fig. 2A, B). Positive autocorrelations (peaks) were observed at distances below 20m, between 45 and 65m and over 90m. At other lag distances, samples were negatively autocorrelated (troughs). The distance between successive peaks (period) was thus T= 50m as indicated by the highest peak in corresponding periodograms (Fig. 2C, D). In transect B, a second large peak was observed at T= 100m. Bromeliad density periodograms showed a different periodicity in transects A and B (Fig. 2G, H). In transect A, we observed four large peaks corresponding to periods of 66.6m, 22.2m, 16.6m and 11.8m. In transect B, we observed a single peak corresponding to a period of 100m. The shape of bromeliad density correlogram of transect B corresponded to a gradient spatial structure, i.e. autocorrelation values decreased with increasing intervals. Periodic spatial structures were observed in the distribution of 10 out of 11 frequent ant species (all but Pheidole flavens, Fig. 3) (Table 2). A strong (example of B. physogaster; Fig. 3A, D) and an intermediate periodicity (example of Solenopsis sp. 17; Fig. 3B, E) were observed in the spatial distribution of four and six species respectively. Solenopsis sp.01, Brachymyrmex physogaster, Wasmannia sp. prox. auropunctata, Octostruma rugifera and Pyramica denticulata showed the same periodicity (example of B. physogaster on Fig. 3D) as litter weight (Fig. 2C, D) with the highest peak at a period of 50m. All frequent species but Crematogaster sp.02 and Paratrechina sp. 02 showed a positive autocorrelation for distance lags below 10m (Fig. 3, example for 3 species). Environmental variation and spatial structure of ant species distribution First, we verified whether the periodicity of leaf litter weight distribution could be related to bromeliad density and vice versa, since the two environmental factors were correlated. Correlograms and periodograms of standardised residuals from the regression between these two factors showed the same highest peak(s) as the initial ones (as in Fig. 2C, D, G, H) although weak variations in periodogram values could be observed. Indeed, we observed no effect of litter weight on the periodicity of bromeliad density. In contrast, bromeliad density influenced periodogram values of litter weight at T= 66.7m (transect A) and at T= 100m (transect B) but had no effect on the highest peak of periodicity at T= 50m (for both transects). In a second step, we evaluated the structuring effect of litter weight and bromeliad density on frequent species distribution with correlograms and periodograms of residuals (e.g. Fig. 4A, B) obtained from the regression between species abundance and the environmental factor considered. Correlograms allowed a visualisation of the decrease of periodicity and periodograms allowed us to quantify it. The percentage of decrease of periodogram values were measured relative to periods where environmental variables showed the highest peak of periodicity, i.e. for litter weight at 50m (transect A and B, Fig. 2C, D) and for bromeliad density at 66.7m (transect A, Fig. 2G) and 100m (transect B, Fig. 2H). The litter weight was a structuring factor for seven frequent ant species as well as the bromeliad density for two of them (Table 2). Litter quantity and bromeliad density as strong structuring factors of ant spatial distribution The structuring effect of environmental variable on each species spatial distribution was explored by inspecting the periodograms of standardised residuals between the abundance of a species and the value of the variable. A strong structuring effect was evident when a peak of abundance of a species experienced a decrease in amplitude over 50%. For example, the peak at a period of 50m in the periodogram of Brachymyrmex physogaster abundance decreased from 7.28 (Fig. 3 D) to 1.89 in the periodogram of residuals (74 % decrease) (Fig. 4B) indicating that leaf litter quantity had a strong structuring effect on Brachymyrmex physogaster distribution. The comparison between the correlogram of a species and of the residuals allowed assessing the structuring effect of an environmental variable (e.g. Fig. 3A and Fig. 4A). The structuring effect of litter weight on the distribution of the two most frequent ant species was strong in both transects (Table 2). In contrast, a strong structuring effect of litter weight was only observed in transect B for Wasmannia sp. prox. auropunctata, Crematogaster sp. 02, Octostruma rugifera, Hypoponera sp. prox. trigona and Pyramica denticulata. The structuring effect of bromeliad density on the distribution of Solenopsis sp. 01 was only strong in transect A. Litter quantity and/or bromeliad density as intermediate structuring factors of ant spatial pattern The analysis of correlograms and periodograms of residuals showed that litter weight had an intermediate spatial structuring effect (20-50% decrease of peaks) on the distribution of Wasmannia sp. prox. auropunctata in transect A. Bromeliad density had an intermediate spatial structuring effect on the spatial distribution of Solenopsis sp.01 (transect B) and Brachymyrmex physogaster (transect A), although the latter was not significantly correlated to bromeliad density (Table 2). In addition, a positive autocorrelation remains at short distance (below 10m) in the correlograms of residuals demonstrating a strong or intermediate structuring effect of leaf litter weight or bromeliad density on species distribution (Fig.4). Species not structured by litter quantity or bromeliad density Two frequent species (Pa. sp. 02 and Ph. nubila) were not structured by the leaf litter weight as determined by a residual analysis although they were correlated to this factor. In the same way, Pa. sp.02 abundance was correlated to, although not spatially structured by, bromeliad density. Discussion Effects of environmental factors vs. interspecific interaction on ant species density and composition Our results suggest that most of the frequent ant species coexist in leaf litter. Indeed, numerous species foraged in the same quadrat (up to 16 species m-2) and 16 positive vs. a single negative associations between frequent species suggested low interspecific competition in our assemblage where foraging ranges may overlap considerably. These results are in agreement with those of previous works (Levings, 1983; Levings and Windsor, 1984; Byrne, 1994; Kaspari, 1996a, b). Moreover, the only negative association was found between two Solenopsis species, which probably occupied very close ecological niches. Weak interspecific competition could be explained by sufficiency of nesting sites and food (Herbers, 1989; Kaspari, 1996b; Soares and Shoereder, 2001) or by avoidance behaviours between heterospecific individuals allowing a high overlap in food utilisation (Byrne, 1994). On the ground, as opposed to the canopy, numerically dominant ants (mostly generalist in our study) do not form a mosaic of non-overlapping territories. The distribution of frequent species of our assemblage was principally associated to leaf litter quantity, rather than competition. Several studies have highlighted the dominant influence of such environmental factors on tropical litter ant assemblages (Franks, 1982; Byrne, 1994; Kaspari, 1996a, b; Soares and Shoereder, 2001). Leaf litter provide nesting sites (Vasconcelos, 1990; Didham, 1998), favorable moisture content (Levings, 1983; Vasconcelos, 1990; Bestelmeyer, 1997), and food resources (Andersen, 1983) for ants and other arthropods (Bestelmeyer and Schooley, 1999a). We observed, as in other studies, a positive correlation between the litter quantity and ant density (Vasconcelos, 1990; Kaspari, 1996b) and composition (Kaspari, 1996b; Carvalho and Vasconcelos, 1999). However several studies did not find an effect of the leaf litter quantity on ant species density and species abundance (Soares and Shoereder, 2001; Delabie and Fowler, 1995). Litter quantity was found to be positively related to litter structural complexity, because of vertical layering (Vasconcelos, 1990). Litter samples displayed variable vertical stratification, some being mainly composed of intact leaves, others of leaves at more advanced stages of decomposition. Vertical litter stratification may allow an increase in the number of coexisting species of ground-dwelling arthropods through habitat partitioning (Anderson, 1978; Vasconcelos, 1990) and by limiting competition (Yanoviak and Kaspari, 2000). Seventy percent (8 out of 11) of frequent species were positively correlated with litter weight. These species could occupy sub-layer(s) of litter composed of decayed leaves and might be specialized to exploit a thick cover of leaf litter. Among species not correlated with litter quantity, we found Crematogaster sp.02 which is arboreal, Pheidole flavens which has the ability to use different microhabitats as nesting sites with some preference for pieces of wood (Wilson, 2003) and Solenopsis sp.17 whose biology is unknown. Bromeliad density was also related to species density and abundance of several ant species but on the whole the impact of bromeliads on the ant assemblage was more limited than the effect of leaf litter quantity (Table 2). Bromeliad leaves form a rosette accumulating rain and litter, and contribute to favorable moisture and temperature conditions for most arthropods (Benzing 1980). Moreover, their spiny leaves provide protection against predators such as opossums, giant anteaters, tamanduas or armadillos (Pujalte et al., 1995; Eisenberg and Redford, 1999). In the same habitat, soil termite diversity is also positively correlated to bromeliad density (Roisin and Leponce, 2004). Spatial pattern of environmental variables We observed variation in litter quantity between contiguous quadrats up to 25 fold, which is consistent with the results obtained elsewhere in the tropics (Kaspari, 1996b). The present study suggests a periodic distribution of the leaf litter. A possible explanation for this phenomenon would be related to topographic differences (microrelief). In another Chacoan Schinopsis balansae forest, Barberis et al. (1998) have demonstrated that most woody species and bromeliads grow preferentially on well-drained convex zones of the soil. The clumped distribution of trees would induce an accumulation of leaf litter on the slightly higher zones whereas the leaf litter would tend to be carried away by temporary inundations in the depressed zones of the forest. Bromeliads, preferring convex zones, tend to increase the quantity of litter possibly because they affect the litter composition, adding their own dead material, and accumulation, due to their root network (Benzing, 1980). This might explain why we observed that some peaks of periodicity of litter quantity could be attributed to bromeliad density. Unfortunately, the periodicity of convex zones remains to be demonstrated. Nevertheless it seems a reasonable hypothesis since periodic pattern of vegetation are sometimes observed (e.g. tiger bush in semi-arid African landscapes, Couteron and Lejeune, 2001). The bromeliad density was also spatially structured but differently so in each transect. We observed a periodic structure in transect A with a period (T= 66.6m) close to that of litter quantity. A gradient was observed in transect B (T= 100m). Gradient structure (Legendre and Fortin, 1989; Legendre and Legendre, 1998) was probably an artefact (false gradient) caused by the presence of a gap, deprived of bromeliads, inside transect B. This trend was also weakly expressed in the leaf litter correlogram (Fig. 2B). Bromeliads showed strong SA below 5m in both transects. This could be a consequence of the asexual reproduction by rhizomes (Benzing, 1980). Structuring effect of environmental variables on the spatial distribution of ants Among the eight species whose abundance was correlated to leaf litter weight, six were strongly spatially structured (period around 50m) by this environmental factor in at least one transect. Structuring effects were generally more apparent in transect B because the ant activity was increased by more favourable temperature conditions. Solenopsis sp. 01 was correlated to and structured by bromeliad density in both transects. The correlation between species abundance and a factor is not necessarily spatial, and may be observed at the quadrat scale without implying a structuring spatial effect of the factor at a larger scale. Conversely, the presence of a structuring effect does not necessarily imply a strong local correlation: species abundance and a factor can fluctuate together at large scale (when the whole transect is considered), but still show a loose association when observed for each quadrat. Two examples illustrate this observation. First, Crematogaster sp.02 (in transect B) was not correlated to litter weight and was found to be distributed with a period of 50m. Its highest peaks of abundance occurred in zones of high litter quantity so that a structuring effect of this factor was detected. Second, Ph. nubila was locally correlated to leaf litter quantity, but not structured by this factor along the whole transect: this species was concentrated mostly at the end of the transects and thus could not be spatially structured by the leaf litter quantity with a 50m period. After removing the structuring effect of the environmental factors (with residual analysis), some peaks of periodicity (at periods different from those that corresponded to our environmental factor effects) persisted indicating that other factors structured the species distribution. These factors could be predation by army ants (Franks and Bossert, 1983; Kaspari, 1996b), other biotic factors (e.g. competition, prey availability), abiotic factors (e.g. soil characteristics, nest-site availability), or stochastic events. Nine out of the 11 frequent species showed a strong spatial structure in their distribution below 10 meters (as shown in Figs. 3 and 4). In other words, species displayed a clumped distribution. The correlogram of residuals (Fig. 4A), indicated that leaf litter quantity was not the cause of this pattern, even for species strongly structured by litter quantity. It is likely that this pattern would be related to the size of the foraging area of individual colonies (Brühl et al., 2003; Delabie et al., 2000b; Kaspari, 1993, 1996b) or to nest aggregation in suitable zones (Herbers, 1989; Soares and Shoereder, 2001). Peaks of species abundance represented in Fig. 1 may indicate the location of nests and gaps between them could reflect intraspecific competition. This would be in agreement with several studies showing that intraspecific interactions affect nest spacing (Levings and Franks, 1982; Ryti and Case, 1984, 1986, 1988, 1992). Dispersal or other environmental factors may also be partly responsible of this pattern. Species showing no spatial structure could be either randomly distributed (Leponce et al., 2004) or could be submitted to several structuring factors with opposing forces. Conclusions Our results suggest that in the subtropical forest studied, the spatial distribution of leaf litter ants is determined at two different scales. At a small scale (period below 10m) a periodic spatial structure is likely to be related to intraspecific competition since we observed, for the most frequent species, a succession of peaks of abundance separated by gaps reducing aggression between allocolonial individuals. At a larger scale (period around 50m), environmental factors, also periodically distributed, may induce aggregates of colonies of species responding positively to these factors. A high quantity of leaf litter and, to a lesser extent, a high density of bromeliads promoted a high density and a high species richness of ants. Interspecific competition, even between numerically dominant ants, was weak. All ant species correlated to an environmental factor were not obligatorily spatially structured by it, suggesting that some other factors, such as intraspecific competition, dipersal and/or environmental factors not measured may have countereffects. Acknowledgments We thank the Administración de Parques Nacionales, Buenos Aires, Argentina, for allowing us to collect in P.N. Río Pilcomayo. Nestor Sucunza, the guardaparques and Cornelio Paredes greatly facilitated our work in the park. Thanks to G.J. Torales and E.R. Laffont, Univ. Nacional del Nordeste, for logistic support. This work was supported by fellowships from the ‘Fonds National de la Recherche Scientifique’ (FNRS, Belgium) to MG and to LT (PhD Grant). A grant to LT from the ‘Fonds Léopold III pour l’Exploration et la Conservation de la Nature’ allowed a field campaign in Argentina. We would like to thank also J.H.C. Delabie and I.C. do Nascimiento (CEPEC, Brasil) for help in ant identification, I. Bachy (RBINS) for help in image treatment, A. Franklin (RBINS) for useful advice in geostatistics, Prof. J.M. Pasteels for critical reading and improvement of the manuscript. ILLUSTRATIONS Fig. 1. Spatial distribution of canopy openness (CO), bromeliad density (BD), leaf litter weight (LW) and distribution of abundance of the four most frequent species (Solenopsis sp.01, Brachymyrmex physogaster, Wasmannia sp. prox. auropunctata and Crematogaster sp.02) along transect A. Black line corresponds to smoothed curves calculating mobile mean of data. Fig. 2. Spatial analysis (correlograms and periodograms) of litter weight (above: A,B,C,D) and bromeliad density (below: E,F,G,H) for transects A (A,C,E,G) and B (B,D,F,H). Highest peaks in periodograms indicate a periodicity of environmental variables. Litter weight was distributed with a 50m period in each transect. Bromeliad density showed different periodicity in his spatial distribution between transects (see text for more details). Rho (h) is the coefficient of autocorrelation varying between -1 and +1. Fig. 3. Periodicity categories of spatial distribution of frequent ant species. Example of correlograms (above A,B,C) and periodograms (below F,G,H) of species showing a strong (A,D), an intermediate (B,E) and a lack of periodicity (C,F) in their spatial distribution along the transect B. The degree of periodicity was estimated according to the amplitude of the highest principal peak of the periodogram and was categorized as either strong (highest peak >6), intermediate (highest peak >1) or none (highest peak <1). Rho (h) is the coefficient of autocorrelation varying between -1 and +1. Fig. 4. Measure of structuring effect intensity of environmental factors (leaf litter quantity and bromeliad density) on frequent ant species distribution. Correlogram (A) and periodogram (B) of residuals from the linear regression between Brachymyrmex physogaster abundance (log (n+1)transformed) and leaf litter weight in transect B. Periodic spatial structure of species distribution disappeared after removing (by regression) leaf litter effects. Rho (h) is the coefficient of autocorrelation varying between -1 and +1. Fig. 1 Fig. 2 Strong Periodicity Fig. 3 Intermediate Periodicity Absence of Periodicity Fig. 4 Table 1: Square matrix with Pearson’s correlation coefficients between abundance of individuals (log10-transformed) of the eleven species taken by pair (transects pooled, N= 320). Statistically significant positive or negative associations between species are greyed or blackened respectively. Levels of significance were adjusted first using Dutilleul's modified t-test and then using Holm’s procedures. Infrequent species were discarded because too little data was available to draw conclusions. Occurrence Rank Species 1 2 3 4 5 6 7 8 9 10 1 Solenopsis sp.01 2 Brachymyrmex physogaster 0.49 1 3 Wasmannia sp. prox. auropunctata 0.37 0.32 1 4 Crematogaster sp.02 0.07 0.07 0.06 1 5 Octostruma rugifera 0.35 0.29 0.15 0.09 1 6 Hypoponera sp. prox. trigona 0.32 0.33 0.26 0.08 0.26 1 7 Paratrechina sp.02 0.26 0.08 0.16 0.02 0.16 0.06 1 8 Pyramica denticulata 0.21 0.16 0.16 0.25 0.29 0.37 0.15 1 9 Solenopsis sp. 17 -0.30 0.12 -0.01 0.03 0.10 0.16 0.04 0.10 1 10 Pheidole flavens 0.07 -0.01 0.07 0.17 0.07 -0.01 0.02 0.01 0.04 1 11 Pheidole nubila 0.07 0.15 -0.01 0.08 0.24 0.31 0.02 0.22 0.24 0 11 1 1 Table 2: Effect of environmental factors on the distribution of frequent ant species (LW = litter weight and BD = bromeliad density). Pearson’s correlation coefficients between the abundance of frequent species (log10-transformed) and environmental factors (raw data) are indicated for pooled quadrats from the 2 transects (N = 320). Levels of significance were adjusted using Dutilleul's modified t-test and Holm’s procedures. Species were sorted by decreasing rank of occurrence in the two pooled transects. Periodicity in species spatial distribution were measured on periodograms and were categorized as either none (highest peak <1), intermediate (highest peak 1-6) or strong (highest peak >6). Between brackets, the periodicity of highest peak was noted. The structuring effect of leaf litter weight and bromeliad density on species spatial distribution corresponded to the percentage of decrease of the 50m (LW) and 66.7 m and 100m (BD) periodical peak and was categorized as either none (decrease <20%), intermediate (decrease 20-50%) or strong (decrease>50%) and were analysed for each transect separately (N= 160). Frequent species Environmental factors Transect A LW BD Periodicity Solenopsis sp.01 0.49 *** 0.31 *** Brachymyrmex physogaster 0.51 *** Wasmannia sp. prox. auropunctata Crematogaster sp.02 Transect B Structuring effect Periodicity Structuring effect LW (50m) LW (50m) BD (66.7m) strong (50m) strong strong strong (66m) strong intermediate 0.16 strong (50m) strong intermediate strong (50m) strong none 0.33 *** 0.06 strong (50m) intermediate none strong (18m) strong none 0.17 0.06 none intermediate (5.25m) strong Octostruma rugifera 0.47 *** 0.11 none none intermediate (50m) strong none Hypoponera sp. prox. trigona 0.47 *** 0.09 intermediate (12.5m) none none none strong (100m) strong none Paratrechina sp.02 0.27 *** 0.28 *** intermediate (13.3m) none none intermediate (10m) none Pyramica denticulata 0.33 ** -0.02 none intermediate (50m) strong none Solenopsis sp. 17 0.23 0.07 intermediate (22m) intermediate (66m) none none Pheidole flavens 0.10 0.07 none none Pheidole nubila 0.34 *** 0.00 none intermediate (33m) none none none none BD (100m) none none Appendix 1. List of species found in transect A and B. Numbers represent their occurrences in the 160 samples collected in each transect. Subfamily Species Transect A Transect B DOLICHODERINAE Linepithema group humile sp.2 0 9 ECITONINAE Eciton vagans 0 3 Labidus coecus 0 3 FORMICINAE Brachymyrmex physogaster 89 95 Brachymyrmex sp.05 (AR) 2 20 Camponotus (Myrmothrix) renggeri 5 0 Camponotus arboreus 2 0 Camponotus crassus 15 23 Camponotus sp. 19 (AR) 0 1 Camponotus sp.11 (AR) 3 1 (Myrmosphincta) Camponotus sp.13 (AR) 0 1 (?Myrmaphaenus) Camponotus sp.14 (AR) 0 2 Camponotus sp.17(AR) 1 0 (Pseudocolobopsis) Myrmelachista sp.02 (AR) 1 7 Paratrechina pubens 4 5 Paratrechina sp.02 (AR) 48 47 MYRMICINAE Acromyrmex hispidus fallax 2 13 Apterostigma sp.complex pilosum 3 10 Carebarella bicolor 3 3 Cephalotes minutus 6 13 Crematogaster corticicola 5 2 Crematogaster euterpe 0 6 Crematogaster montezumia 2 0 Crematogaster sp.02 (AR) 28 78 Crematogaster sp.07 (AR) 1 1 Crematogaster sp.11 (AR) 1 6 Crematogaster sp.14 (AR) 2 0 Crematogaster sp.16 (AR) 0 2 Cyphomyrmex rimosus 10 13 Leptothorax sp.01 (AR) 0 8 Leptothorax sp.02 (AR) 0 2 Megalomyrmex drifti 1 5 Mycocepurus goeldii 0 2 Myrmicocrypta foreli 0 2 Octostruma rugifera 39 66 Oxyepoecus reticulatus 1 1 Pheidole aberrans 11 2 Pheidole nubila 17 37 Pheidole sp.01 (AR) 23 34 Pheidole sp.04 (AR) 9 0 Pheidole sp.21 (AR) 0 2 Pheidole sp.22 (AR) 12 47 Pheidole sp.30 (AR) 7 40 Pyramica crassicornis 2 1 Pyramica denticulata 21 81 Pyramica gr. appretiata sp.01 (AR) 2 0 Pyramica gr. appretiata sp.02 (AR) 0 2 Pyramica sp.02 (AR) 8 4 Rogeria scobinata 10 24 Solenopsis clytemnestra bruchi 0 1 Solenopsis sp. 17 (AR) 20 46 Solenopsis sp. 18(AR) 5 6 Solenopsis sp.01 (AR) Solenopsis sp.02 (AR) Solenopsis sp.10 (AR) Solenopsis sp.13 (AR) Solenopsis sp.15 (AR) Strumigenys louisianae Strumigenys ogloblini Strumigenys sp. prox.elongata 1 Trachymyrmex sp.01 (AR) Wasmannia sp. prox. auropunctata Wasmannia sp.03 (AR) PONERINAE Amblyopone sp.01 (AR) Anochetus diegensis Discothyrea neotropica Ectatomma edentatum Ectatomma permagnum Gnamptogenys striatula Heteroponera sp.01 (AR) Hypoponera clavatula Hypoponera opaciceps Hypoponera opacior Hypoponera sp. 09 (AR) Hypoponera sp. prox. opaciceps 1(AR) Hypoponera sp. prox. trigona Hypoponera sp.04 (AR) Hypoponera sp.05 (AR) Hypoponera sp.07 (AR) Leptogenys consanguinea Odontomachus chelifer Odontomachus meinerti Pachycondyla ferruginea Pachycondyla harpax Pachycondyla obscuricornis Pachycondyla villosa Prionopelta punctulata Typhlomyrmex pusillus PSEUDOMYRMECI Pseudomyrmex gracilis NAE 101 11 0 5 3 0 1 1 0 66 4 1 4 8 13 0 4 3 1 4 6 0 1 29 29 1 0 2 4 1 1 4 0 1 1 1 3 98 15 6 1 5 2 0 4 7 102 4 0 2 0 19 1 6 0 0 12 18 2 5 66 3 1 1 1 5 0 1 9 5 0 2 1 0 REFERENCES Adams, E.S., 1994. Territory defence by the ant Azteca trigona - maintenance of an arboreal ant mosaic. Oecologia 97: 202-208. Andersen, A.N., 1983. Species diversity and temporal distribution of ants in the semi-arid mallee region of northwestern Victoria. Aust. J. Ecol. 8: 127-137. Anderson, J.M., 1978. Inter- and intra-habitat relationships between woodland cryptostigmata species diversity and the diversity of soil and litter microhabitats. Oecologia 32: 341-348. Barberis, I.M., E.F. Pire and J.P. Lewis, 1998. Spatial heterogeneity and woody species distribution in a Schinopsis balansae (Anacardiaceae) forest of the Southern Chaco, Argentina. Rev. Biol. Trop. 46: 515-524. Benson, W.W. and C.R.F. Brandão, 1987. Pheidole diversity in the humid Tropics: a survey from Serra dos Carejas, Para, Brazil. In: Chemistry and Biology of Social Insects (J. Eder and H. Rembold, Eds), München, Verlag J. Peperny, pp. 593-594. Benzing, D.H., 1980. The Biology of the Bromeliaceae. Mad River Press Inc., Eureka, California, 305 pp. Bestelmeyer, B.T., 1997. Stress tolerance in some Chacoan dolichoderine ants, implications for community organisation and distribution. J. Arid Envir. 35: 297-310. Bestelmeyer, B.T., 2000. The trade-off between thermal tolerance and behavioural dominance in a subtropical South American ant community. J. Anim. Ecol. 69: 998-1009. Bestelmeyer, B.T. and R.L. Schooley, 1999. The ants of the southern Sonoran desert: community structure and the role of trees. Biodivers. Conserv. 8: 643-657. Bestelmeyer, B. and J.A. Wiens, 2001. Local and regional-scale responses of ant diversity to a semi-arid biome transition. Ecography 24: 381-392. Brühl, C.A., T. Eltz and K.E. Linsenmair, 2003. Size does matter - effects of tropical rainforest fragmentation on the leaf litter ant community in Sabah, Malaysia. Biodivers. Conserv. 12: 1371-1389. Byrne, M.M., 1994. Ecology of twig-dwelling ants in a wet lowland tropical forest. Biotropica 26: 61-72. Carvalho, K.S. and H.L. Vasconcelos, 1999. Forest fragmentation in central Amazonia and its effects on litter-dwelling ants. Biol. Conserv. 91: 151-157. Couteron, P. and O. Lejeune, 2001. Periodic spotted patterns in semi-arid vegetation explained by a propagation-inhibition model. J. Ecol. 89: 616-628. Delabie, J.H.C. and H.G. Fowler, 1995. Soil and litter cryptic assemblages of Bahian cocoa plantations. Pedobiologia 39: 423-433. Delabie, J.H.C., D. Agosti and I.C. do Nascimento, 2000a. Litter ant communities of the Brazilian Atlantic rain forest region. In: Sampling Ground-dwelling Ants: Case Studies from the Worlds' Rain Forests (Agosti, D., J. Majer, L.E. Alonso and T. Schultz, Eds), Curtin University, Australia, School of Environmental Biology, Bulletin no 18, pp.1-17. Delabie, J.H.C., S. Campiolo and D. Fresneau, 2000b. Etude comparative de la saturation des communautés de fourmis des litières sous latitudes tropicales et tempérées. Actes Coll. Insectes Soc. 13: 71-75. Devillers, P. and J. Devillers-Terschuren, 1996. A Classification of South American Habitats. IRSNB, Brussels, and Institute of Terrestrial Ecology, Huntingdon, 419 pp. Didham, R.K., 1998. Altered leaf-litter decomposition rates in tropical forest fragments. Oecologia 116: 397-406. Dutilleul, P., 1993., Modifying the t test for assessing the correlation between two spatial processes. Biometrics 49: 305-314. Eisenberg, J.F. and K.H. Redford, 1999. Mammals of the Neotropics: the central Neotropics, Volume 3. The University of Chicago Press: 609 pp. Feener, D.H. and E.W. Schupp, 1998. Effect of treefall gaps on the patchiness and species richness of Neotropical ant assemblages. Oecologia 116: 191-201. Fisher, B.L., 1998. Ant diversity patterns along an elevational gradient in the Réserve Spéciale d'Anjanaharibe-Sud and on the Western Masoala Peninsula, Madagascar. Fieldiana Zool. (n.s.) 90: 39-67. Franks, N.R., 1982. Social insects in the aftermath of swarm raids of the army ant Eciton burchelli. In: The Biology of Social Insects (Breed, M.D., C.D. Michener and H.E. Evans, Eds). Westview Press, Boulder, CO, pp. 275-279. Franks, N.R. and W.H. Bossert, 1983. The influence of swarm raiding army ants on the patchiness and diversity of tropical leaf litter ant community. In: Tropical Rain Forest: Ecology and Management (Sutton, E.L., T.C. Whitmore and A.C. Chadwick, Eds), Blackwell, Oxford, pp. 151-163. Frazer, G.W., C.D. Canham and K.P. Lertzman, 1999. Gap Light Analyzer (GLA), Version 2.0: Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-color Fisheye Photographs, Users Manual and Program Documentation. Copyright 1999: Simon Fraser University/Institute of Ecosystem Studies, Burnaby, BC/ Millbrook/NY. Gadakgar, R., P. Nair, K. Chandrashekara and D.M. Bhat, 1993. Ant species richness and diversity in some selected localities in western Ghats, India. Hexapoda 5: 79-94. Herbers, J.M., 1989. Community structure in north temperate ants: temporal and spatial variation. Oecologia 81: 201-211. Hirosawa, H., S. Higashi and M. Mohamed, 2000. Food habits of Aenictus army ants and their effects on the ant community in a rain forest Borneo. Insect. Soc. 47: 42-49. Höfer, H., C. Martius and L. Beck, 1996. Decomposition in an Amazonian rain forest after experimental litter addition in small plots. Pedobiologia 40: 570-576. Holm, S., 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6: 65-70. Jaccard, P., 1912. The distribution of flora in the alpine zone. New Phytol. 11: 37-50. Jackson, D.A., 1984. Ant distribution patterns in a Cameroonian cocoa plantation: investigation of the ant mosaic hypothesis. Oecologia 62: 318-324. Kaspari, M., 1993. Body size and microclimate use in Neotropical granivorous ants. Oecologia 96: 500-507. Kaspari, M., 1996a. Litter ant patchiness at 1-m² scale: disturbance dynamics in three Neotropical forests. Oecologia 107: 265-173. Kaspari, M., 1996b. Testing resource-based models of patchiness in four Neotropical litter ant assemblages. Oikos 76: 443-454. Kaspari, M. and J.D. Majer, 2000. Using ants to monitor environmental changes. In: Ants: Standard Methods for Measuring and Monitoring Biodiversity (Agosti, D., J. Majer, L.A. Alonso and T. Schultz, Eds), Smithsonian Institution Press. pp. 89-98. Legendre, P. and M.-J. Fortin, 1989. Spatial pattern and ecological analysis. Vegetatio 80: 107-138. Legendre, P. and L. Legendre, 1998. Numerical Ecology. Second English edition. Elsevier, Development in environmental modelling, 20: 853 pp. Legendre, P., M.R.T. Dale, M.-J. Fortin, J. Gurevitch, M. Hohn and D. Myers, 2002. The consequences of spatial structure for the design and analysis of ecological field surveys. Ecography 25: 601-615. Lennon, J. J., 2000. Red-shifts and red herrings in geographical ecology. Ecography 23: 101–113. Leponce, M., L. Theunis, J.H.C. Delabie and Y. Roisin, 2004. Scale dependence of diversity measures in a leaf-litter ant assemblage. Ecography, 27: 253-267. Levings, S.C., 1983. Seasonal, annual and among-site variation in the ground ant community of a deciduous tropical forest: some causes of patchy species distributions. Ecol. Monogr. 53: 435-455. Levings, S.C. and N.R. Franks, 1982. Patterns of nest dispersion in a tropical ground ant community. Ecology 63: 338-344. Levings S.C. and D.M. Windsor, 1984. Litter moisture content as a determinant of litter arthropod distribution and abundance during the dry season on Barro Colorado Island, Panama. Biotropica 16: 125-131. Liebhold, A.M. and J. Gurevitch, 2002. Integrating the statistical analysis of spatial data in ecology. Ecography 25: 553-557. Liebhold, A.M., R.E. Rossi and W.P. Kemp, 1993. Geostatistics and geographic information systems in applied ecology. Annu. Rev. Entomol. 38: 303-327. Moutinho, P.R.S., 1998. Impactos do uso da terra sobre a fauna de formigas, consequências para recuperação florestal na Amazônia Oriental. In: Floresta Amazônica: dinâmica, regeneração e manejo (Gascon, C. and P. Moutinho, Eds), Manaus, MCT-INPA, pp. 155-170. Perry, J.N., A.M. Liebhold, M.S. Rosenberg, J. Dungan, M. Miriti, A. Jakomulska and S. CitronPousty, 2002. Illustrations and guidelines for selecting statistical methods for quantifying spatial pattern in ecological data. Ecography 25: 578-600. Pujalte, J.C., A.R. Reca, A. Balabusic, P. Canevari, L. Cusato and V.P. Fleming, 1995. Anales de parques nacionales. Unidades Ecológicas del Parque Nacional Río Pilcomayo. Administración de Parques Nacionales XVI: 1-185. Retana, J. and X. Cerdà, 2000. Patterns of diversity and composition of Mediterranean ant communities tracking spatial and temporal variability in the thermal environment. Oecologia 123: 436-444. Roisin, Y. and M. Leponce, 2004. Characterizing termite assemblages in fragmented forests: a test case in the Argentinian Chaco. Austral Ecol. 29: 637-646. Rossi, R.E., D.J. Mulla, A.G. Journel and E.H. Franz, 1992. Geostatistical tools for modelling and interpreting ecological spatial dependence. Ecol. Monogr. 62: 277-314. Ryti, R.T. and T.J. Case, 1984. Spatial arrangements and diet overlap between colonies of desert ants. Oecologia 62: 401-404. Ryti, R.T. and T.J. Case, 1986. Overdispersion of ant colonies: a test of hypotheses. Oecologia 69: 446-453. Ryti, R.T. and T.J. Case, 1988. The regeneration niche of desert ants: effects of established colonies. Oecologia 75: 303-306. Ryti, R.T. and T.J. Case, 1992. The role of neighbourhood competition in the spacing and diversity of ant communities. Am. Nat. 139: 355-374. Shumway, R. H., 1988. Applied Statistical Time Series Analysis. - Englewood Cliffs, NJ: Prentice Hall, pp. 117-165. Soares, S.M. and J.H. Schoereder, 2001. Ant nest distribution in a remnant of tropical rainforest in southeastern Brazil. Insect. Soc. 48: 280-286. StatSoft, Inc., 2004. STATISTICA (data analysis software system), version 6. www.statsoft.com. Vasconcelos, H.L., 1990. Effects of litter collection by understory palms on the associated macroinvertebrate fauna in Central Amazonia. Pedobiologia 34: 157-160. Vasconcelos, H.L. and J.H.C. Delabie, 2000. Ground ant communities from central Amazonia forest fragments. Sample Ground-dwelling ants: Case studies from the Worlds' Rain Forests. Curtin University School of Environmental Biology Bulletin, Perth, Australia 18: 59-70. Vasconcelos, H. L., A. C. C. Macedo and J. M. S. Vilhena. 2003. Influence of topography on the distribution of ground-dwelling ants in an Amazonian forest. Stud. Neotrop. Fauna Environ. 38: 115-124. Wilson, E.O., 1958. Patchy distribution of ant species in New Guinea rain forests. Psyche 65: 2638. Wilson, E.O., 2003. Pheidole in the New World: A dominant, hyperdiverse Ant Genus. Harvard University Press, Cambridge, Massachusetts, 794 pp. Wilson, M.V and A. Shmida, 1984. Measuring beta diversity with presence-absence data. J. Ecol. 72: 1055-1064. Yanoviak, S. and M. Kaspari, 2000. Community structure and the habitat templet: ants in the tropical forest canopy and litter. Oikos 89: 259-266. CHAPITRE II Effects of habitat type on ground-dwelling ant assemblage in a fragmented forest of the humid Chaco. LAURENCE THEUNIS 1, 2, YVES ROISIN 2, JACQUES H.C. DELABIE 3 AND MAURICE LEPONCE 1. 1. Section of Conservation Biology, Royal Belgian Institute of Natural Sciences, Rue Vautier 29, B-1000 Brussels, Belgium, e-mail: Laurence.Theunis@naturalsciences.be 2. Behavioral and Evolutionary Ecology, CP 160/12, Université Libre de Bruxelles, Avenue F.D. Roosevelt 50, B-1050 Brussels, Belgium 3. Centro de Pesquisas do Cacau (CEPEC-CEPLAC), 45600-000 Itabuna, Bahia and Departamento de Ciências Agrárias e Ambientais, Universidade Estadual de Santa Cruz, 45660-000, Ilhéus, Bahia, Brazil Laurence Theunis Phone +32 2 627.43.64 Fax +32 2 649.48.25 E-mail: Laurence.Theunis@naturalsciences.be RUNNING HEAD: EFFECTS OF HABITAT TYPE ON ANTS. ABSTRACT INTRODUCTION Fragmented landscapes are composed of a patchwork of habitats of differing quality for fauna. It is obvious that understanding how species are distributed in a fragmented forest requires information on their responses to all components of the landscape i.e. forest fragments and the matrix (Malcolm 1991, Laurance 1994). Ants are considered to be good bioindicators (Andersen 1990, King et al. 1998, Lobry de Bruyn 1999) because of their abundance, diversity and function across a range of habitats in all trophic levels. They play important functions in ecosystems such as nutrient cycling, seed dispersal and the population regulation of other insects (Bestelmeyer and Wiens 2003, Hölldobler and Wilson 1990, Folgarait 1988). Moreover, ant assemblages usually respond to habitat modifications and disturbances (Majer et al. 1997, Suarez et al. 1998, Carvalho and Vasconcelos 1999, Brühl et al. 2003, Vasconcelos and Delabie 2000, Soares and Schoereder 2001). Fire is one of the most common disturbances occurring in grasslands that can possibly maintain boundaries with forest fragments and cause large scale and dramatic changes in species diversity (Farji-Brener et al. 2002, McCullogh et al. 1998, Abbott 1984). However, the effects of fire on ant assemblages are less well known. Farji-Brener et al. (2002) showed that prairiedwelling ants were, in some measure, capable of surviving fire so that fire-related changes for ants were small and short-term because of rapid regeneration of the xeric steppe. Fire possibly may play a beneficial role in some ecosystems where species recovery is rapid preventing monopolization of limiting resources by dominant species (Panzer 1998, Farji-Brener et al. 2002). The National Park of Río Pilcomayo constituted (one of) the largest area protected of the Argentinian humid Chaco, offering so a system at maturity of a subtropical forest fragmented amidst by a grassland. Such grassland regularly submitted to natural floods and burns contained ant species showing excellent strategies of resistance facing to these natural regular disturbances (Levieux, 1972, Pujalte et al., 1995). Regular fires in the natural grassland of the NPRP generally did not decrease and could even increase the species diversity (Vogl 1974, Frangi et al., 1982). The present study aimed to compare first the ant assemblages between the forest fragments and the grassland and then to estimate the impact of recent fires on the ant assemblage in the grassland. MATERIAL AND METHODS STUDY AREA We carried out the study in the Río Pilcomayo National Park situated in the humid Chaco of the north-eastern Argentina (25°04’06’’ S, 58°05’36’’ W). Average annual rainfall in the park is about 1200mm, with a short dry period (0-3 months) in the southern winter, between June and September. Temperature fluctuates broadly, with an annual average of 22-24°C and occasional winter frost (Pujalte et al. 1995). The park is a mosaic of vegetation types, depending primarily on inundation frequency. The present study was limited to the semidecideous forest fragments (Monte Fuerte) displaying a considerable degree of fragmentation and the grassland (Pastizal) regularly submitted to natural floods and fires (Morello, 1970). The forest was dominated by Schinopsis balansae Engl., Astronium balansae Engl. and Aspidosperma quebracho-blanco Schlecht. and by a ground strata of bromeliads (Aechmea distichantha Lemaire and Pseudananas sagenarius (Arruda) Camargo) (Pujalte et al. 1995). The grassland was dominated by herbaceous vegetation as Setaria sp., Luziola peruviana and palm trees as Copernicia alba (Pujalte et al., 1995). During the autumn 2001 and 2002, a great part of the grassland of the study area experienced extensive fires, which could not penetrate into the forest fragments moister than the matrix. Therefore, a mosaic including both burned and unburned sites were delimited. SAMPLING PROCEDURE During the autumn of 2001 and of 2002, we sampled ants in the grassland and in two large forest fragments (250ha). We conducted a 500m long transect in each fragment with increasing distance from the forest edge and five 200m-long transects in the grassland. Three transects were conducted in the grassland recently burned and two in the grassland unburned. We sampled ants in the grassland burned from 5 to 15 days after fire. Ants were captured using pitfall traps put 10m apart along transects for 6 days. We filled them, every two days, with diluted alcohol (ethanol). DATA ANALYSES Ants were identified to species or alternatively to morphospecies. Species richness, number of species by pitfall and species composition were estimated to compare ant assemblages between the two habitats. Because of its strong dependence of sample size and species density, species richness must be standardizing before comparisons. Species richness difference between communities was thus calculated by bootstrapping using the PAST software (Hammer et al. 2004). Faunal similarity between habitats was assessed using the incidence-based Jaccard estimator (Chao et al. 2005). This new similarity index increased accuracy of the measure but also avoided the underestimation of similarity occurring because of the failure to account for unseen shared species (species that are likely to be present in a larger homogeneous sample of the assemblage, but that are missing from actual sample data). Numerically dominant species (species present in at least 10% of samples) were compared using their frequencies in the sample. Species were also classified in functional group (Andersen and Majer 2000, Delabie et al. 2000). Comparison of ant assemblage between the grassland and the forest Ant assemblages (species richness, number of species by pitfall traps and species composition) were compared between the grassland (5 transects of 20 pitfalls) and the forest of the two large fragments (2 transects of 50 pitfalls). Fire effects on ant assemblage in the grassland Ant assemblages (species richness, number of species by pitfall traps and species composition) were compared between the grassland recently burned (3 transects of 20 pitfalls) and the grassland no submitted to recent fire (2 transects of 20 pitfalls). RESULTS COMPARISON OF ANT ASSEMBLAGE BETWEEN THE GRASSLAND AND THE FOREST Ant species richness was not different between the grassland and the forest (Table 1). Species density collected by pitfall traps was greater for the grassland than for the forest (Table 1). Faunal similarity between the forest and the grassland was 0.62 (Incidence-based Jaccard). Five species were commonly frequent in the two habitats, seven species were frequent in the grassland and rarer in the forest, five others species were frequent in the grassland and absent from the forest fragments. Finally, five species were frequent in the forest and rarer in the grassland (Fig. 1). Fungus-grower ant as Acromyrmex hispidus fallax or litter predators as Pachycondyla striata and Gnamptogenys striatula were more frequent in the forest habitat. In the grassland, we collected a lot of species omnivores moving on the vegetation or on the ground as Camponotus sp.14 (AR), C. sp.15 (AR), C. crassus, Crematogaster sp.18 (AR) and Paratrechina pubens. FIRE EFFECT ON THE GRASSLAND ANT ASSEMBLAGE The species richness and activity of ants was similar between the grassland recently burned and unburned. Faunal similarity between the grassland recently burned and unburned was 0.61 (± 0.11) (Incidence-based Jaccard) (Table 1). The grassland recently burned had more frequent species: 19 vs. 12 species. Six of them were absent in the grassland unburned (Fig. 2). Dominance in unburned sites was composed of some species highly frequent. In contrast, in burned sites, the dominance was represented by a greater number of species with moderate frequencies. Considering the ant functional group, we noted that the arboreous omnivores ant foraging on the floor Crematogaster sp.18 (AR) was preferentially collected in the grassland unburned. Predators species, as Odontomachus meinerti, Ectatomma edentatum and E. permagnum, were more frequent in the grassland burned than in the grassland unburned. DISCUSSION COMPARISON OF ANT ASSEMBLAGE BETWEEN THE GRASSLAND AND THE FOREST In spite of the great difference of the vegetation type and structure between the grassland and the forest, 77% of the frequent ant species were sampled in both habitat types, indicating that a relative high proportion of the frequent ant species are ubiquist i.e. adapted to these two contrasted habitats. Nevertheless, five species were exclusively collected in the grassland. Despite of his severe climatic conditions, the grassland had a higher number of species by pitfall and a larger number of frequent ground-dwelling species (17 vs. 10) than in the forest. Among them, genera as Camponotus, Brachymyrmex, Pogonomyrmex and Linepithema were well represented in the grassland. Camponotus sp. 15 dominated numerically the grassland with a frequency in the pitfall of 54%. No ground dwelling ant species were exclusively collected in the forest. Indeed, pitfall traps allowed capturing principally species moving on the ground. As a consequence, the ground myrmecofauna of the forest, principally constituted by a lot of small and cryptic leaf-litter ant species (as Solenopsis sp. 01 and S. sp 18), required others collecting methods as Winkler 24hr to obtain data more representative of his assemblage (Leponce et al. 2004, Theunis et al., accepted). However, the leaf-litter of the forest rich in arthropods favoured the presence of the predator ant as Pachycondyla striata and Gnamptogenys striatula. The species Acromyrmex hispidus fallax was also typical of the forest habitat where they find the leaves necessary to the growing of their fungus. FIRE EFFECT ON THE GRASSLAND ANT ASSEMBLAGE In the grassland, recent fires modified the native ant assemblage principally about his species composition. Indeed, the species richness and the number of ant by pitfall trap were similar in both burned and unburned sites. In contrast, the faunal similarity indicated that ant assemblage in the grassland recently burned or not were different as much as between the grassland and the forest. Differences in ant assemblage composition after burning were reported in several studies (Andersen 1991, MacKay et al., 1991; Andrew et al. 2000, York 2000). Six frequent species were exclusively collected in burned sites. Moreover, more frequent species (19 vs 12 sp.) was collected in recently burned grassland and their frequencies were more equilibrated than in the unburned sites i.e. 13 frequent species in the burned site had a frequency between 20 and 45%. Comparatively, in unburned grassland, among the twelve frequent species, the half had a frequency superior than 30% and the other close to 10%. First, common frequent species to burned and unburned sites are probably capable of surviving fire (Shoereder at al. 2004). The vegetation of the grassland was so dry that fire burned it rapidly sparing some ant colonies nesting in the soil. Second, fires could re-equilibrate populations of dominant colonies allowing to pioneers species to recolonize the habitat before the monopolisation by few dominant species of the exploitation of the resources (as we saw in unburned grassland) (Panzer 1998, Farji-Brener et al. 2002). A greater number of frequent species in burned site could be allowed probably with a temporal resource partitioning based upon differences in thermal tolerances, limiting so interspecific competition for foraging areas (Bestelmeyer 2000, Andersen, 1991; Delsinne?). The interaction of temperature and competitive relationships explain a great deal about the structure of many ant assemblages (Andersen, 1995; Hölldobler and Wilson 1990). Fires, by reducing plant cover, increase the availability of open habitats that are ideal for heat-tolerant ant species (Andersen, 1991, 1992). Bestelmeyer (2000) showed, in the Chaco, that behaviourally dominant ants were most active at moderately high temperatures, whereas subordinate species were active at extreme temperatures (heat tolerant species), when they had virtually exclusive access to resources. Several species could forage during the night avoiding in this way the extreme heat of the day (Betselmeyer 2000, Andersen 1986). Finally, Betch and Cancela de Fonseca (1995) showed that nutrients are often released after burning and promote the development of some arthropod communities attracting different ants. It is possible that predator species, as Odontomachus meinerti, Ectatomma edentatum and E. permagnum, were attracted by the great arthropod biomass appearing after fires and had an important role in the organization and function of arthropod assemblages (Castaño-Meneses and Palacios-Vargas 2003, Kaspari 2001). Our results thus showed ant species could survive to fires and even that fires could have beneficial role to the ant assemblage offering to some species the possibility to colonize the habitat. Nevertheless, we know that the grassland is also submitted to annual inundations lasting to 2-3 months (Ramella and Spichiger, 1989). Floods must induced larger modifications in ant assemblage of the grassland than fires. Forest fragments could constitute a reservoir for some ant species common to both habitats recolonising the grassland in the dry season (ref). However, the species must suffer a drastic decrease in their population size. As a consequence, species exclusively collected in the grassland (5 species) have to develop other strategies than find refuge in the soil for their survival in case of inundation. Very few data on this phenomenon are available in the literature. Some Formica queens could survive under water at least 14 days and they could be transported by water floating until they reach new island habitat (Gyllynberg and Rosengren, 1984). Studies conducted in the NPRP showed that regular fires in the natural grassland generally did not decrease and could even increase the species diversity (Vogl 1974, Frangi et al., 1982). Such grassland regularly submitted to natural burns contained ant species showing excellent strategies of resistance facing to this natural regular disturbance as for others cryptic species like termites, armadillos, some amphibian (Levieux, 1972, Pujalte et al., 1995). In contrast, sessile colonies of ant must be dramatically affected by inundation because of an abrupt decrease of favourable habitat availability. The size of ant population in the grassland must thus vary extremely along years with his extremes between dryness and inundation (Pujalte et al. 1995). Acknowledgments – We thank the Administración de Parques Nacionales, Buenos Aires, Argentina, for allowing us to collect in P.N. Río Pilcomayo. Nestor Sucunza, the guardaparques and Cornelio Pares greatly facilitated our work in the park. Thanks to G.J. Torales and E.R. Laffont, Univ. Nacional del Nordeste, for logistic support. This work was supported by the ‘Fonds National pour la Recherche Scientifique’ (FNRS, Belgium) to LT (PhD Grant). A grant to LT from the ‘Fonds Léopold III pour l’Exploration et la Conservation de la Nature’ allowed a field campaign in Argentina. We would like to thank also J. H. C. Delabie and I. C. do Nascimiento (CEPEC, Brasil) for help in ant identification, I. Bachy (RBINS) for help in image treatment and Prof. J.M. Pasteels for useful comments on manuscript. REFERENCES Abbott I. (1984) Changes in the abundance and activity of certain soil and litter fauna of the Jarrah forest of Western Australia after moderate intensity fire. Australian Journal of Soil Research 22: 463470. Andersen A.N. (1990) The use of ant communities to evaluate change in Australian terrestrial ecosystems: a review and a recipe. Proceedings of the Ecological Society of Australia 16: 347-357. Andersen A.N. (1991) Responses of ground-foraging ant communities to three experimental fire regimes in a savanna forest of tropical Australia. Biotropica 23: 575-585. Andersen A.N. (1992) Regulation of “momentary” diversity by dominant species in exceptionally rich ant communities of the Australian seasonal tropics. American Naturalist 140: 401-420. Andersen and Majer 2000 Andrew N., Rodgerson L. and York A. (2000) Frequent fuel-reduction burning: the role of logs and associated leaf litter in the conservation of ant biodiversity. Australian Ecology 25: 99-107. Bestelmeyer B. (2000) The trade-off between thermal tolerance and behavioral dominance in a subtropical South American ant community. Journal of Animal ecology 69: 998-1009. Bestelmeyer B.T., Wiens J.A. (2003) Scavenging ant foraging behavior and variation in the scale of nutrient redistribution in semiarid grasslands. Journal of Arid Environments 53(3): 373-386. Betch J.M. and Cancela da Fonseca P. (1995) Changes in edaphic factors and microarthropod communities after clearing and burning in a tropical rain forest in French Guyana. Acta Zoologica Fennica 196: 142-145. Brühl C.A., Eltz T. and Linsenmair K.E. (2003) Size does matter – effects of tropical rainforest fragmentation on the leaf litter ant community in Sabah, Malaysia. Biodiversity and Conservation 12: 1371-1389. Carvalho K.S. and Vasconcelos H.L. (1999) Forest fragmentation in central Amazonia and its effects on litter-dwelling ants. Biological Conservation 91: 151-157. Castaño-Meneses G. and Palacios-Vargas J.G. (2003) Effects of fire and agricultural practices on neotropical ant communities. Biodiversity and Conservation 12: 1913-1919. Chao A. ; Chazdon R.L.; Colwell R.K. and Shen T.-J. (2005) A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecology Letters 8: 148-159. Delabie J.H.C.; Agosti D. and do Nascimento I.C. (2000). Litter ant communities of the Brazilian Atlantic rain forest region. In: Sampling Ground-dwelling Ants: Case Studies from the Worlds' Rain Forests (Agosti, D.; Majer, J.; Alonso, L.E. and Schultz, T. Eds), Curtin University, Australia, School of Environmental Biology, Bulletin no 18, pp.1-17. Farji-Brener A. G., Corley J.C. and Bettinelli J. (2002) The effects of fire on ant communities in north-western Patagonia: the importance of habitat structure and regional context. Diversity and Distribution 8: 235-243. Folgarait P. (1988) Ant biodiversity and its relationship to ecosystem functioning: review. Biodiversity and Conservation 7: 1221-1244. Frangi J.L. Ronco M.J., Sanchez N.E., Vicari R.L. and Rovetta G.S. (1982) Efecto del fuego sobre la composición y dinámica de la biomasa de un pastizal de Sierra de la Ventana (Bs. As., Argentina). Darwiniano 22 : 565-585. Gyllynberg G. and Rosengren R. (1984) The oxygen consumption of submerged Formica queens (Hymenoptera, Formicidae) as related to habitat and hydrochoric transport. Annales Entomologici Fennici 50: 76-80. Hölldobler B. and Wilson E.O. (1990) The ants. Cambridge University Press, Cambridge. Kaspari M. (2001) Taxonomic level, trophic biology and the regulation of local abundance. Global Ecology and Biogeography 10: 229-244. King J.R., Andersen A.N. and Cutter A.D. (1998) Ants as bioindicator of habitat disturbance: validation of the functional group model for Australia’s humid tropics. Biodiversity and Conservation 7: 1627-1638. Laurance 1994 Leponce et al. 2004, Levieux, 1972, viens de pujalte mais oubli de mettre dans sa biblio!!! Lobry de Bruyn L.A. (1999) Ants as bioindicators of soil function in rural environments. Agriculture, Ecosystem and Environment 74: 425-441. MacKay W., Rebeles A., Arredondo H., Rodriguez A., González D. and Vinson B. (1991) Impact of slashing and burning of a tropical rain forest on the native ant fauna (Hymenoptera: Formicidae). Sociobiology 18: 257-268. Majer J.D., J.H.C. Delabie and N.L. McKenzie (1997. Ant litter fauna of forest, forest edges and adjacent grassland in the Atlantic rain forest region of Bahia, Brazil. Insectes Sociaux 44: 255-266. Malcolm 1991 McCullogh D.G., Werner R.A. and Neumann D. (1998) Fire and insect in northern and boreal forest ecosystems of North America. Annual Review of Entomology 43: 107-127. Morello J. (1970) Ecología del Chaco. Boletín de la Sociedad Argentina de Botánica Vol XI (supl.): 161-174. Panzer 1998 Pujalte J.C., A.R. Reca A. Balabusic P. Canevari L. Cusato and Fleming V.P. (1995) Anales de parques nacionales. Unidades Ecológicas del parque nacional Rio Pilcomayo. Administración de Parques Nacionales XVI: 1-185. Ramella L. and Spichiger R. (1989) Interpretación preliminary del medio físico y de la vegetación del Chaco Boreal – Contribución al studio de la flora y de la vegetación del Chaco. I. Candollea 44 : 639-680. Shoereder J.H., Sobrinho T.G., Ribas C.R. and Campos R.B.F. (2004) Colonization and extinction of ant communities in a fragmented landscape. Austral Ecology 29: 391-398. Soares and Schoereder 2001 Theunis et al., accepted Vasconcelos and Delabie 2000, Vogl R.J. (1974) Fire and Ecosystem. Kozlovski T.T. and Ahlgreen C.E., Eds, Academy Press Inc. USA. York A. (2000) Long-term effects of frequent low-intensity burning on ant communities in coastal blackbutt forests of southeastern Australia. Austral Ecology 25: 83-98. Table 1: Ant assemblage (species richness, number of species by pitfall trap and faunal similarity) comparison between the forest and the grassland and between the grassland recently burned and unburned. Forest Grassland Number of samples 100 100 60 40 Number of occurrences 413 504 324 180 Species richness 59 57 42 Bootstrap Number of species by pitfall trap Incidence-based Jaccard Similarity Index Burned 38 ns 4 (0-11) Unburned ns 5 (1-13) 5.4 (0 - 13) 4.5 (0 - 9) U= 3486 ** U= 1058.5, p= 0.3208 0.62 (± 0.1) 0.61 (± 0.11) Figure 1: Frequency in the samples of species in the grassland and forest. We used the frequent species i.e. which were present in at least 10% of the samples (black narrow). Species were collected by pitfall traps. The functional group of the species was put in brackets and corresponded as follows: A= arboreous omnivores foraging on the floor, B= fungus-grower, using vegetation, C= epigeaic and litter omnivores, D= epigeaic and litter seeds specialists and omnivores, E= epigeaic and litter generalist predators, F= epigeaic and litter specialist predators, and G= hypogeaic specialist predators. Figure 2: Frequency in the samples of species in the grassland recently burned and unburned. We used the frequent species i.e. which were present in at least 10% of the samples (black narrow). Species were collected by pitfall traps. The functional group of the species was put in brackets and corresponded as follows: A= arboreous omnivores foraging on the floor, B= fungus-grower, using vegetation, C= epigeaic and litter omnivores, D= epigeaic and litter seeds specialists and omnivores, E= epigeaic and litter generalist predators, F= epigeaic and litter specialist predators, and G= hypogeaic specialist predators. Effects of the presence of terrestrial bromeliads on leaf litter ants in a Chacoan forest. LAURENCE THEUNIS 1, 2, YVES ROISIN 2 AND MAURICE LEPONCE 1. 1. Section of Conservation Biology, Royal Belgian Institute of Natural Sciences, Rue Vautier 29, B-1000 Brussels, Belgium. 2. Behavioral and Evolutionary Ecology, CP 160/12, Université Libre de Bruxelles, Avenue F.D. Roosevelt 50, B-1050 Brussels, Belgium. Laurence Theunis Phone +32 2 627.43.64 Fax +32 2 649.48.25 E-mail: Laurence.Theunis@naturalsciences.be Running head: Effects of bromeliads on ants. ABSTRACT We previously demonstrated that the litter-dwelling ant species richness and species density was positively correlated to the density of terrestrial bromeliad in patches of forest where they constitute a continuous ground cover. The aim of the present paper was to evaluate the effects of the bromeliad absence on the ant assemblage (species richness, density, frequency and composition). Inside the same forest, located in the humid Chaco region, we compared the ant assemblages inside and outside bromeliad patches. Species richness was 10% higher (57 vs. 52 sp) and the species density doubled in the bromeliad zone. Ant species ranking, based on their frequency in samples, was different between the two zones (rank order Kendall Tau correlation r=0.11, ns) with 15 species specialist in bromeliads zones and only two of zones deprived of them. The high faunal similarity (Chao-Jaccard Incidence based index= 0.96) was probably overestimated because the contiguous zones implied introgression of species between them. Indeed, numerous species specialist of the bromeliads were collected in quadrat contiguous to a bromeliad patch increasing the species richness of zones without bromeliads. The bromeliad patches had a slightly higher leaf litter quantity by samples. The bromeliads constituted thus a micro-habitat essential to the ant assemblage in the forest, and probably for several arthropods, providing water reserve, shade, protection against predators and an abundant litter. INTRODUCTION For ground-dwelling ants, it has been already demonstrated that vegetation structure and composition influence the distribution of ant colonies and could be partly responsible of their patchiness (Wilson, 1958; Gadagkar et al., 1993; Feener and Schupp, 1998; Moutinho, 1998; Retana and Cerdà, 2000; Bestelmeyer and Wiens, 2001). In particular, the presence of terrestrial Bromeliaceae on the ground favours the diversity of several arthropods taxa. For example, they increase the diversity and the density of the jumping spiders (Romero and Vasconcellos-Neto 2004). In a previous study, we investigated the ant species distribution in a forest fragment of the humid Chaco with a continuous ground cover of terrestrial bromeliads (Theunis et al., in press). Our results showed that a high density of these plants promoted a high species density and a high species richness of ants (Theunis et al., in press). Additionaly, bromeliad density was found to increase the abundance of two common ant species and to structure their spatial distribution. Finally, some of us demonstrated that bromeliad patches favoured a higher soil- or interfacefeeding termites diversity (higher species richness and abundance) (Roisin and Leponce 2004). In complement to our results showing the impact of bromeliad density on ant species density and richness in a forest with a continuous bromeliad cover, our goal here was to evaluate the impact of the bromeliad absence on the structure of the ant assemblage in terms of faunal composition, species richness, species density and species frequency. This comparison was made possible by the existence of large patches deprived of terrestrial bromeliads in the same forest. MATERIAL AND METHODS STUDY AREA We carried out the study in the Rio Pilcomayo National Park situated in the humid Chaco of the north-eastern Argentina (25°04’06’’ S, 58°05’36’’ W). The park is a mosaic of vegetation types, depending primarily on inundation frequency. The present study was limited to the semideciduous forest (Monte Fuerte), which occupies 20-22% of the park area (Pujalte et al. 1995) and displays a high degree of natural fragmentation amidst by grassland. This forest was dominated by Schinopsis balansae Engl., Astronium balansae Engl. and Aspidosperma quebracho-blanco Schlecht. and by a ground strata of bromeliads (Pseudananas sagenarius (Arruda) Camargo) continuously or patchily distributed (Pujalte et al. 1995). SAMPLING PROCEDURE Leaf-litter ants were collected along 500m linear transects perpendicular to the edge of large forest fragments of approximately 250ha. Three parallel transects, distant of 10m, were conducted in October 2001 in a forest fragment where the ground-cover of bromeliads was patchily distributed. Another transect was conducted in October 2002 in a large fragment with a continuous bromeliad cover. Each transect consisted of 50 quadrats of 1m² separated by 10m intervals. At each sampling point, the leaf litter found inside the 1m²-quadrat was collected, sifted and put in a cotton bag. The sifted material was brought back to field laboratory and its fauna was extracted with a mini-Winkler apparatus (Fisher, 1998) for 24 hours. Leaf litter samples were weighted with an electronic scale before extraction (data only available for the 150 samples collected in 2001). Since the data sets collected in 2001 (n=35 samples) and 2002 (n=50) in bromeliad patches did not differ (same incidence-based rarefaction curves, same species density and high faunal similarity index), they were pooled (n=85). DATA ANALYSES Ant assemblages inside or outside bromeliads zones were compared on basis of their species richness, density and composition. Species richness was standardized by incidence-based rarefaction (Coleman method of EstimateS 7.5 (Colwell 2004)). Species density (number of species/m²) was compared between the two forest zones using a Mann-Whitney U test. We also compared the leaf litter quantity between zones inside and outside bromeliads. Compositional similarity of ant assemblages was assessed using the incidence-based Chao-Jaccard estimator (Chao et al. 2005) which takes into account unseen shared species of assemblages not exhaustively inventoried. Frequent species (defined as species present in more than 10% of samples) were compared using their frequency in samples. First, we tested if the ranking of species, based on their frequency, was the same inside and outside of bromeliad patches (Kendall Tau correlation test). Then, we tested if the presence of each frequent species was independent of the bromeliad presence (Chi² tests). RESULTS Standardized species richness was 10% higher inside than outside bromeliad patches (57 vs. 52 species, respectively). The density of species was two-fold greater inside than outside bromeliad patches (Median (min-max): 6 (0-14) vs. 3 (0-13), Mann-Whitney U test: U= 2627.5, p< 0.0001). Median leaf litter weight was marginally different (U= 1574, p= 0.06, n1= 35, n2= 115) inside (240 g (min: 99 - max: 751)) and outside (210 g (60-1184)) patches. Faunal similarity between the two forest types was 0.96 (±0.04) (Incidence-based Chao-Jaccard). We found 22 and 9 species frequent in samples inside and outside of bromeliad patches, respectively; eight of them were shared (Table 2). Species ranking, based on their frequency in samples, was different inside and outside bromeliad zones (Rank-Order Kendall Tau correlation r= 0.12, ns). Two species were negatively associated with the bromeliad presence: Crematogaster sp.17 (Chi²=8.21, df= 1, p < 0.01) and Solenopsis sp. 18 (Chi²=6.56, df= 1, p < 0.01). Seven species were not influenced by the bromeliad presence. In contrast, out of 22 species frequent in bromeliad patches, 15 were positively associated to bromeliad presence (Table 2). Among them, Rogeria scobinata was absent in zones devoid of bromeliads and 10 others species, infrequent outside bromeliad patches, were collected in quadrats adjacent to a bromeliads zone. DISCUSSION We already showed that the bromeliads density influenced the spatial structure of some ant species distribution inside a patch of bromeliad (Theunis et al., in press). Indeed, a high bromeliad density favored a high abundance of two species (Solenopsis sp. 01 and Brachymyrmex physogaster) and spatially structured the distribution of two others (Solenopsis sp. 01 and Paratrechina sp. 02) (Theunis et al., in press). The present work shows that the bromeliads presence or absence had a larger impact influencing the distribution of whole ant assemblage. Indeed, the ant species richness was higher and the species density doubled in bromeliads patches indicating that most of the ant species colonies are concentrated in the bromeliads. Numerically dominance of ant species was different inside and outside bromeliad patches. Indeed, fifteen species (65% of the frequent species) preferred the bromeliad zones. The spiny leaves of bromeliads provide protection against predators such as opossums, giant anteaters, tamanduas or armadillos (Pujalte et al. 1995, Eisenberg and Redford 1999). Moreover, the complex three-dimensional architecture of the Bromeliaceae could favour the predator species (Gnamptogenys striatula, Octostruma rugifera and Hypoponera sp. prox. Trigona, Hypoponera opacior, Hypoponera sp. prox. opaciceps 01) as it has been shown for jumping spiders (Romero and Vasconcellos-Neto 2004). Finally, bromeliad leaves form a rosette accumulating rain and litter, and contribute to favourable moisture and temperature conditions for most arthropods (Benzing 1980, Romero and Vasconcellos-Neto 2004). Indeed, the litter quantity is positively correlated in zones of high density of bromeliad (Theunis et al., in press) and was, in the present study, marginally higher in bromeliad patches. On the one hand, a greater number of data on the litter weight in the bromeliads would reinforce this result. On the other hand, we could not exclude that the bromeliad presence improved the litter quality (mycorrhizae improving litter decomposition, moisture, etc) and consequently attracted arthropods. Only two species were negatively associated with patches of bromeliad (Crematogaster sp. 17 and Solenopsis sp.18). Unfortunately, informations about the biology of these species do not exist in the literature. The seven others species were not influenced by the bromeliad. Among them, we found species using different micro-habitats (wood, soil, leaf litter) and having a large distribution as Solenopsis sp. 02, S. sp17, Carebarella bicolor, Pheidole flavens and Wasmannia sp.01 or arboreal species as Crematogaster sp.02 and Cephalotes minutus (Wilson 2000, de Andrade?). Numerous species specialist of the bromeliads were collected in quadrats contiguous to a bromeliad patches increasing the species richness of the zones without bromeliads. As a consequence, the faunal similarity (Chao-Jaccard Index= 0.96) was overestimated because this index takes into account unseen shared species of assemblages not exhaustively inventoried. The Jaccard Incidence-based estimator adjusted by Chao et al. (2005) probably unsuited the estimation of faunal similarity of two contiguous zones. In conclusion, the bromeliad zones constituted thus, in the monte fuerte, a favourable microhabitat to the ant assemblage increasing the species richness, doubling species density and containing fifteen species associated to the bromeliads. They probably provided water reserve, shade, protection against predator and abundant litter quantity. References Benzing, D.H., 1980. The Biology of the Bromeliaceae. Mad River Press Inc., Eureka, California, 305 pp. Bestelmeyer, B. and J.A. Wiens, 2001. Local and regional-scale responses of ant diversity to a semi-arid biome transition. Ecography 24: 381-392. Chao, A., R. L. Chazdon, R. K. Colwell, and T.-J. Shen. 2005. A new statistical approach for assessing compositional similarity based on incidence and abundance data. Ecology Letters 8:148-159. Colwell, R. K. 2004. EstimateS: Statistical estimation of species richness and shared species from samples. Ver. 7.5. User’s guide and application published at <http://viceroy.eeb.uconn.edu/estimates>. de Andrade Eisenberg, J.F. and K.H. Redford, 1999. Mammals of the Neotropics: the central Neotropics, Volume 3. The University of Chicago Press: 609 pp. Feener, D.H. and E.W. Schupp, 1998. Effect of treefall gaps on the patchiness and species richness of Neotropical ant assemblages. Oecologia 116: 191-201. Fisher, B.L., 1998. Ant diversity patterns along an elevational gradient in the Réserve Spéciale d'Anjanaharibe-Sud and on the Western Masoala Peninsula, Madagascar. Fieldiana Zool. (n.s.) 90: 39-67. Gadakgar, R., P. Nair, K. Chandrashekara and D.M. Bhat, 1993. Ant species richness and diversity in some selected localities in western Ghats, India. Hexapoda 5: 79-94. Moutinho, P.R.S., 1998. Impactos do uso da terra sobre a fauna de formigas, consequências para recuperação florestal na Amazônia Oriental. In: Floresta Amazônica: dinâmica, regeneração e manejo (Gascon, C. and P. Moutinho, Eds), Manaus, MCT-INPA, pp. 155-170. Pujalte, J.C., A.R. Reca, A. Balabusic, P. Canevari, L. Cusato and V.P. Fleming, 1995. Anales de parques nacionales. Unidades Ecológicas del Parque Nacional Río Pilcomayo. Administración de Parques Nacionales XVI: 1-185. Retana, J. and X. Cerdà, 2000. Patterns of diversity and composition of Mediterranean ant communities tracking spatial and temporal variability in the thermal environment. Oecologia 123: 436-444. Roisin, Y. and M. Leponce, 2004. Characterizing termite assemblages in fragmented forests: a test case in the Argentinian Chaco. Austral Ecol. 29: 637-646. Romero, G.Q. and J. Vasconcellos-Neto 2004. Spatial distribution patterns of jumping spiders associated with terrestrial bromeliads. Biotropica 36: 596-601. THEUNIS, L., M. GILBERT, Y. ROISIN AND M. LEPONCE (IN PRESS). SPATIAL STRUCTURE OF LITTERDWELLING ANT DISTRIBUTION IN A SUBTROPICAL DRY FOREST. INSECTES SOCIAUX. Wilson, E.O., 1958. Patchy distribution of ant species in New Guinea rain forests. Psyche 65: 2638. Wilson, E.O., 2003. Pheidole in the New World: A dominant, hyperdiverse Ant Genus. Harvard University Press, Cambridge, Massachusetts, 794 pp. Table 1: Comparison of ant assemblages inside or outside bromeliad patches. bromeliads No bromeliads Number of samples 85 114 Number of occurrences 534 396 Species richness rarefied 57 52 Species density (number of species by m²) 6 (0-14) 3 (0-13) Incidence-based Jaccard Similarity Index U= 2627.5 *** 0.96 (± 0.04) Table 2: Proportion of samples occupied by each species inside (n= 85) or outside (n=115) bromeliad patches and association of species with bromeliad presence (Chi² test, 1 df). Only species which were present in >10% of the samples collected in any of the two groups compared were considered here. Species in the grey box correspond to species collected outside bromeliad patch but in quadrats contiguous (at 10 meters) to a bromeliad zone. Inside bromeliad Outside bromeliad Association with patches patches bromeliad presence Crematogaster sp.02 (AR) 53 44 no Wasmannia sp.01 (AR) 36 25 no Pheidole flavens (AR) 22 31 no Solenopsis sp.02 (AR) 22 27 no Cephalotes minutus 21 13 no Solenopsis Sp. 17 (AR) 21 15 no Solenopsis Sp. 18 (AR) 18 34 + ** Carebarella bicolor 12 20 no Rogeria scobinata 16 0 + *** Solenopsis sp.01 (AR) 47 6 + *** Pyramica denticulata 44 1 + *** Pheidole sp.30 (AR) 11 2 + ** Brachymyrmex physogaster 25 2 + *** Brachymyrmex sp.05 (AR) 25 1 + *** Octostruma rugifera 22 2 + *** Pheidole nubila 20 1 + *** Pheidole sp.22 (AR) 20 4 + *** Hypoponera sp. prox. trigona 14 1 + *** Gnamptogenys striatula 12 3 + ** Hypoponera opacior 12 4 + * Hypoponera sp. prox. opaciceps 01(AR) 12 3 + ** Paratrechina sp.02 (AR) 35 5 + *** Crematogaster sp.17 (AR) 5 18 ** Species CHAPITRE III Effects of natural forest fragmentation on the structure of a leaf litter ant assemblage in the humid Chaco. LAURENCE THEUNIS 1, 2, YVES ROISIN 2 AND MAURICE LEPONCE 1. 1. Section of Conservation Biology, Royal Belgian Institute of Natural Sciences, Rue Vautier 29, B1000 Brussels, Belgium. 2. Behavioral and Evolutionary Ecology, CP 160/12, Université Libre de Bruxelles, Avenue F.D. Roosevelt 50, B-1050 Brussels, Belgium. Laurence Theunis Phone +32 2 627.43.64 Fax +32 2 649.48.25 E-mail: Laurence.Theunis@naturalsciences.be Running head: Ants in a naturally fragmented forest ABSTRACT INTRODUCTION Because of their ecological importance and sensibility to environmental conditions and changes, ants are precocious indicators of biodiversity (Majer et al. 1984, Agosti et al. 2000b), of disturbance (Majer 1983, Bestelmeyer and Wiens 1996, Andersen 1997, Vasconcelos 1999, Carvalho and Vasconcelos 1999, Brühl et al. 2003) and of ecosystems rehabilitation (Majer et al. 1984, Vasconcelos et al. 2001). Reasons for what some studies focused on this numerically dominant group in forest to evaluate impacts of disturbances caused by forest fragmentation (Vasconcelos 1999, Carvalho and Vasconcelos 1999, Brühl et al. 2003). Literature on fragmentation effects on ant diversity generally occurred in tropical rain forests exposed to a high rate of anthropogenic deforestation as in Central Amazonia (Biological Dynamic of Forest Fragment Project, see Carvalho and Vasconcelos 1999, Didham 1997 a and b), in the Atlantic rainforest (Majer et al. 1997, Delabie et al. 2000) or in the lowland forest in Malaysia (Brühl et al. 2003). Generalizations about the effects of forest fragmentation on ant diversity are difficult because of the large variation of studies conditions as the site, the habitat (history and type), the season and the sampling conditions. However, small and isolated fragment could not contain the original diversity and intact forest contains more species per unit area (species density) than fragments (Vasconcelos 1988, Didham 1997b, Vasconcelos and Delabie 200b, Brühl et al. 2003). There is also evidence that ant species composition in small forest fragments (1 ha) is influenced by the structure and composition of the vegetation surrounding these fragments (Vasconcelos and Delabie 1998). Vasconcelos and Delabie (2000b) demonstrated that the ant composition was generally more affected by the geographic distance than by the fragment size (between 1 and 100 ha). Carvalho and Vasconcelos (1999) showed that the edge effects significantly affected ant species composition and that this effect was partly attributable to variation in litter depth. Ant richness, density and composition were affected by edge effects up to 200m inside Central Amazonian forests (Didham 1997a, Vasconcelos et al. 1998, Carvalho and Vasconcelos 1999). To date no information exists on the structure of ant assemblages in a naturally fragmented forest at maturity. Our aim was to evaluate to which degree species richness, species density and species composition was influenced by the size, shape and isolation of the fragments of a mature (dry) forest Additionally we evaluated the edge effects on ant species density and composition and the introgression of species from the habitat (grassland) surrounding the forest fragments. MATERIAL AND METHODS STUDY AREA The study was carried out in the Rio Pilcomayo National Park, in the wet Chaco region (25°05’ S, 58°08’ W), which is a mosaic of vegetation types, depending primarily on inundation frequency. The park was created in 1951 and left undisturbed since then providing an appropriate framework to study the effects of a fragmented forest upon ants, in terms of low (anterior to 50 years) anthropic intervention, and of landscape pattern. Anthropic interventions before 1951 consisted in wood exploitation and cattle rearing by local human populations. The present study was limited to a dense (“impenetrable”) subtropical mesoxerophile oligarchic forest (Pujalte et al., 1995; habitat unit PHYSIS 48.2412 of Devillers and DevillersTerschuren, 1996), which occupies 20-22% of the park area (Pujalte et al. 1995), and displays a considerable degree of natural fragmentation amidst grassland of herbaceous vegetation and palm trees, called pastizal. The forest fragments (called locally “monte fuerte”) are located on slight mounds and their edges are maintained by both natural fires and floods occurring regularly in the surrounding grassland. The forest is characterised by Schinopsis balansae Engl., Astronium balansae Engl. and Aspidosperma quebracho-blanco Schlecht. and by the presence of a ground stratum of bromeliads (Aechmea distichantha Lemaire and Pseudoananas sagenarius (Arruda) Camargo) (Pujalte et al. 1995). GENERAL ANT SAMPLING PROTOCOL Ants were collected either by Winkler extraction or by pitfalls traps. Samples were collected at intervals of 10 meters along a linear transect. Pitfall traps were only used to compare the ant fauna inhabiting the forest and the grassland. In all other transects we used the Winkler method which consists in collecting the leaf-litter present inside 1m²-quadrats and extracting the ant fauna with a mini-Winkler apparatus (Fisher 1998) during 24 hours. Most of the Winkler transects we conducted corresponds to the standardized 200m transect designed to collect ants of the leaf-litter (“A.L.L.” transect of Agosti & Alonso 2000). In the habitat studied a single standardized A.L.L. transect with 20 Winkler samples, collects less than 45% of the ant species present in the forest fragment (Leponce et al. 2004). All frequent species are included but their relative frequency is not always representative. Ants were collected in September and October during four consecutive years (1999-2002). Temperature during the sampling sessions ranged was on average of 27.7 ± 5.1°C (n= ? days). Effects of size, shape and isolation of the forest fragments We used a SPOT image (1:5,000,000 scale) to select eleven accessible forest fragments belonging to three size categories: small (< 4ha, n=5 fragments, “S1”-“S5”), medium (between 15 and 30ha, “M1”-“M4”) and large (around 250ha, “L1” and “L2”) in three localities (“Esteros”, “Fonzo” and “la Angela”) (Fig. 1). In small and medium forest fragments, A.L.L. transects (200m long) were conducted along the longest axis of the fragment. In large fragments transects were 500 m long and perpendicular to an edge (3 parallel transects, 10m apart, were conducted in “L1” and a single transect was conducted in “L2”). To study the effects of fragment size we only considered samples further than 200m from the edge. Indeed, 200m is the maximal distance at which edgeeffects on invertebrates assemblages (including leaf-litter ants) have been detected (Laurance et al. 2002, Carvalho & Vasconcelos 1999, Didham 1997 a, b). All forest fragments had a dense and continuous ground stratum of bromeliads, except “L1” where these plants were patchily distributed. The bromeliad density affects the ant and termite species distribution (Roisin and Leponce 2004, Theunis et al., in press). In order to validate the inter-fragment differences observed, the intra-fragment variability of our measures was assessed by conducting 8 A.L.L transects (“M*1”-“M*8”) between 1 and 7 Augustus 2000 in the medium fragment “M3” (see Leponce et al. 2004 for details). Edge effects on ant species density and composition Species density and composition were estimated from the edge to the centre of the two large fragments “L1” and “L2”. The variation of species density was studied along the 3 parallel transects of “L1”. Only quadrats devoid of terrestrial bromeliads were considered to avoid any confounding effect of bromeliad density (n= 114 samples). The introgression of grassland species in the forest was studied with pitfall traps spaced at 10m intervals along transects running from the grassland, at 50m of the forest edge, to 500m inside the forest. One 550m transect was run along the central Winkler transect of “L1” and another along the Winkler transect of “L2”. Pitfall traps were left during 6 days and filled with ethanol 50%. The variation of 5 environmental variables from the edge towards the centre of a forest fragment was evaluated in a strip of 500m x 2m along the central Winkler transect in “L1”. The strip was divided in 100 quadrats of 5m x 2m in which the number of trees, the diameter-at breast height of the trees, the number of shrubs, the number of bromeliad rosettes were measured. In addition, the leaf-litter collected in 1m² quadrats every 10m was weighted before Winkler extraction. The variation of species composition from the edge towards the centre of a forest fragment was studied by pooling Winkler and pitfall catches from “L1” (150 Winkler and 50 pitfalls samples) and “L2” (50 Winkler and 50 pitfalls samples). Only frequent species (defined as occurring in >10% of samples) were considered for this analysis which was conducted on species abundance (log10 (x+1)-transformed) in order to limit the weight of samples collected around nests, trails and exploited resources FRAGMENTATION INDICES The area (A), perimeter (P), and distance between fragments was calculated with MapInfo Professional 6.5 (MapInfo Corporation 2001). The shape of fragments was characterized by the ratio logP/logA and by the Shape Index defined by Patton (1975) as P/ (200*(π * A)–1/2), where P is the perimeter of the forest patch in metres, and A is its area in hectares. Its value is 1 for a circle. Values >1 represent deviations from circularity (Laurance and Yensen 1991, Magura et al. 2001). Isolation of fragments was measured as the distance to the nearest fragment (NF) and with the mean nearest fragment distance (MNFD) calculated on all neighbour patches within 600 m around the studied forest fragment. The radius of 600 m was chosen because even poor colonist forest specialist can cover this distance through inhospitable habitats (Magura et al. 2001). Isolation of a habitat patch depends not only on the distance on the nearest patch, but also of the area of the nearest patch. For that reason, we used a third isolation measure called the Proximity Index (PI) (Gustafson and Parker 1994). It is calculated, for all patches within the 600m radius, as PX= Σ An/dn2, where An is the area of the neighbour patch and dn is the distance between the patch and its neighbour. We then divided PX by the mean distance between the patch considered and all neighbour patches. DATA ANALYSIS Size, shape and isolation of forest fragments For all forest fragments, the Pearson correlation coefficient was calculated between the values of species density, species richness and the 7 fragmentation indices. We then evaluated impact of fragmentation on ant species composition. Species richness The measure of species richness is strongly dependent of the number of samples collected and of the species density during the sampling period (Gotelli and Colwell 2001, Leponce et al. 2004). To standardise and compare the species richness among the different fragments we relied on the Melo’s method (Melo et al. 2003) which compares the values of species richness expected for the largest occurrence (232 occurrences in our case) among all transects. This method avoid to loose information by comparing the species richness expected for the smallest occurrence (58 occurrences in our case) among all transects with the traditional rarefaction method of Sanders (1968). In Melo’s method occurrence-poor datasets are extrapolated with an appropriate curvefitting extrapolation model. In a preliminary study, some of us (Leponce et al., in revision) observed that the choice of the best performing extrapolation method depended of the pattern of species accumulation obtained during the inventory. For inventories still showing a logarithmic pattern of species accumulation, the Soberón and Llorente logarithmic model (S=ln (1+z*a*x)/z) where S is the number of species and a, b, z are fitted coefficients performed best (Soberón and Llorente 1993, Fisher 1999, Leponce et al. 2004). We used the Longino’s method to analyse the logarithmic or asymptotic pattern of curves (Longino 2002) For inventories which attain a more asymptotic pattern of species accumulation, the Stout and Vandermeer (1975) asymptotic model (S=a/[x^z+(a/b)] where a, b, z are fitted coefficients) performed best. Accordingly either a Soberón and Llorente or a Stout and Vandermeer model was used to extrapolate the curves. Nevertheless, we also rarefied the species richness following the rarefaction method (Saunders 1968) and the results leaded us to the same conclusions. We conserved thus only the results obtained by the Melo’s extrapolation method which avoid the loose of data. Accumulation curves of species richness S (± SD) for each fragment was realised using the EstimateS 7.0 program (Colwell 1994-2004). Curve-fitting models were applied on accumulation curves with the non-linear estimation procedure and the quasi-Newton estimation method in Statistica 5.0 (StatSoft Inc 2004). The degree of significance of the differences in species richness was calculated by bootstrapping using in PAST software (Hammer et al. 2001). Species density We used the mean species density calculated with 20 (small and medium fragments) or 30 Winkler samples (large fragments). Species composition The comparison of species the ant composition between forest fragments was conducted by Non-Metric Multidimensional Scaling (NMDS). Non-Metric Multidimensional Scaling (NMDS) is at present the recommended non-linear ordination method for community analysis (Minchin 1987, Carvalho and Vasconcelos 1999, Vasconcelos 1999, Vasconcelos et al. 2000, Golden & Crist 2000, Brühl 2003). This technique preserves, as well as possible, the distance relationships among object and can produce ordinations of objects from any distance matrix (Legendre and Legendre 1998). The Bray-Curtis faunal similarity index calculated on species frequencies (Majer et al. 1997) was used in the NMDS. NMDS was calculated with PAST version 1.29 (Hammer et al. 2001). NMDS has already been applied in ant studies and produced robust results (Carvalho & Vasconcelos 1998, 1999, Golden & Crist 2000, Vasconcelos 1999, Vasconcelos et al. 2000). A cluster analysis (UPGMA) was then superimposed upon the resulting spatial map to indicate the fragments which were most similar in terms of ant species composition. We tested the differences between clusters using an ANOVA using the ordination scores obtained with the NMDS. RESULTS SPECIES INVENTORY CHARACTERISTICS Altogether 112 species (I= 3787 occurrences, n= 40350 individuals) were collected with the Winkler method and 83 species (I= 1214 occurrences, n= 4277 individuals) with pitfall traps. One hundred twenty-seven species were collected by both methods. Species accumulation curves in fragments S2, S5, M2, M3, M4, L1 and L2 failed to approach a plateau (Table 1). In contrast, species accumulation curves in S1, S3, S4 and M1 were more asymptotic (Table 1). The difference between the species richness values among the fragments were presented in Table 2. EFFECTS OF FRAGMENTATION INDICES ON SPECIES RICHNESS AND SPECIES DENSITY Species density was correlated with none of the fragmentation indices (Table 3). Differences of species density could not be attributed to temperature variations (Pearson’s r= 0.33, p= 0.16). Standardized species richness was positively correlated to the area (r= 0.60, p <0.05), the perimeter (r= 066, p< 0.05), the Shape Index (r= 081, p< 0.01) and marginally with the Proximity Index (r= 0.56, p= 0.07). A Kruskal-Wallis test showed a significant difference of the standardized species richness (for 232 occurrences) between the 3 sizes of forest fragments (KW = 8.430, p=0.0379) (Fig. 2). A Dunn's Multiple Comparisons Test revealed a significant difference (P<0.05) between the small forest fragments and the control EFFECT OF FRAGMENTATION INDICES ON ANT SPECIES COMPOSITION The NMDS ordination of faunal similarities was presented on the Figure 3. Transects from the calibration transect were closely aggregated and were located close to transect conducted 10 month later in the same fragment M3. Ant composition split into three clusters at 50 and 60 % of similarity: cluster I with S1, S2, M1 and M4. S3, S4 and S5 were grouped into cluster II and M*, M2, M3, L1 and L2 were grouped into cluster III. The three clusters were significantly different from each other along dimension 1 (ANOVA: df= 2, F= 22,882, p< 0.0001; Tukey post-hoc test: cluster I - II ***, cluster I - III * and cluster II - III ***) and along dimension 2 (ANOVA: df= 2, F= 58, 504, p< 0.0001; Tukey post-hoc test: cluster I - II ***, cluster I - III *** and cluster II - III ns). Ant species composition was affected by fragment isolation. This was shown by the significant relationship between the scores produced by the first axis of the ordination analysis (NMDS) and the nearest fragment distance (Pearson’s r= -0.46, p< 0.05) and the mean nearest fragment distance (r= -0.47, p< 0.05). NMDS scores for the second axis of the ordination analysis was correlated with the Proximity Index (r= -0.46, p< 0.05). Nevertheless, some species were collected in forest fragments belonging to a particular size; i.e. Brachymyrmex sp. 05 and Hypoponera sp.prox. opaciceps 1 were frequent only in large fragments and Crematogaster sp.05 was absent from this size category. Camponotus renggeri was collected exclusively in small forest fragments and Pheidole nubila, Rogeria scobinata and Solenopsis sp. 18 were frequent species in medium and large ones only. EDGE EFFECTS Species density measured in the 3 parallel Winkler transects in L1 was not different between the edge and the centre of the forest fragment (Unpaired t-test, F= 1.241, p= 0.22) (Fig. 4). The number of species by pitfall did not vary as a function of distance to fragment edge. However, at edge in L1, species density was elevated between 0 and 60m (Fig. 4). Twenty-eight frequent ant species were frequent either in the large forest fragments or the grassland (Table 4). Three species were collected in both the grassland and at the edge of the forest fragment (Crematogaster sp.18, Paratrechina pubens and Pheidole aberrans). Four were encountered only in the grassland (Hypoponera sp.08, Paratrechina sp.01, Solenopsis sp. 14 and S. Sp. 16) and three others only in the center of the forest fragment (Brachymyrmex sp. 05, Paratrechina sp. 02 and Pyramica denticulata). Most of the frequent species (11/24) were leaf-litter specialists without preference for the edge or the center of the forest. In contrast, the abundance (log transformed) of three species was higher near the edge than near the centre (Carebarella bicolor, Crematogaster sp.11 and Solenopsis sp.18). Finally, we collected four ubiquist species: Camponotus crassus, Labidus preadator, Pheidole sp.21 and Wasmannia sp.01. None of the environmental factors measured (the number of trees, the diameter-at breast height of the trees, the number of shrubs, the number of bromeliad rosettes) varied significantly between the edge and the centre of the fragments. Nevertheless, the bromeliads were patchily distributed along L1. DISCUSSION (SPECIES INVENTORY CHARACTERISTICS AND EXTRAPOLATED SPECIES RICHNESS) Small fragments presented either a logarithmic accumulation curve but with lower extrapolated species richness (S1, S2 and S5, bootstrapping) than medium and large ones or an asymptotic accumulation curve that reached a plateau indicating the complementarity (or nearly) of the species inventory (S1, S3 and S4). Medium fragments showed a high variation in their species richness; two were closer to small fragments characteristics (M1 and M2) and the others were more similar to the large ones which showed high species richness and a logarithmic accumulation curve. Large fragments were rare in the study site limiting the number of fragments for this size category. As a consequence, differences of species richness extrapolated between size categories (Fig. 2) of forest fragments were significative only between small fragments and the control; although small fragments had an obvious tendency to be less rich in ant species number than other categories of fragments size. The difficulty with fragmented forest studies is always that the available forest fragments are not situated in a way that would allow perfect replication of plots with exactly the same environmental and sampling conditions. In order to limit the ‘noise’, we sampled all fragments available and accessible to obtain results as representative as possible of this natural fragmented forest. Extrapolation for the maximal common occurrence was an adequate and reliable method allowing the use of all available data and limiting the errors related to a too large extrapolation from small size sample. Melo et al. (2005) suggested that extrapolations are safe up to a double sample size of the data in study. We have shown that some physical characteristics of the forest fragments influenced the structure of leaf-litter ant communities in this naturally fragmented forest. In particular, species richness was correlated to the size, the shape and the isolation degree of fragments. In contrast, species density was not correlated with the fragmentation indices. Although, climatic conditions (temperature and moisture) determined the activity of ground-dwelling ants (Bestelmeyer, Delsinne, Leponce et al. 2004, Levings?), the low temperature variation during our sampling periods did not influence species density values. Finally, we have shown that the species composition was influenced principally by the fragment isolation. EFFECTS OF FRAGMENT SIZE Below 20ha (medium fragments), fragments contained a lower ant species richness. Our system followed the prediction of the theory of island biogeography (Mac Arthur and Wilson 1967); i.e. fragment size and isolation reduced species richness (Brühl et al. 2003…). Although there was a high difference of area between medium and large fragments, larger forest fragments did not improve the ant species richness. Brühl et al. (2003) observed the same trends in their studies. However, we observed difference of ant composition between fragment sizes, i.e. some species were exclusively collected in fragments of particular size. For example, two species (B. sp. 05 and H. sp.prox. opaciceps 1) were collected exclusively in large fragments and were centre specialists. EFFECTS OF FRAGMENT SHAPE Interestingly, species richness was higher in fragments with elevated Shape Index (edge-area ratio of the fragment) than in fragments close to circularity. Edge conditions probably allowed increasing the structural heterogeneity of the fragment and consequently providing more niche space in the fragment for invertebrates in particular (Kotze and Samways 2001, Williams-Linera 1990). Because the species density was not influenced by the shape index, the increase of species richness in fragments with high edge-area ratio must be due to a high ant species turnover between quadrats. Discuss edge effects in large!! EFFECTS OF FRAGMENT ISOLATION Fragments isolation tended to decrease the species richness. The higher the distance from neighbor fragments the less the alates could cross the grassland to recolonize a fragment. The composition of the ant assemblage was influenced principally by the degree of isolation of the fragments. Fragments were separated in 3 clusters (by NMDS) concerning their ant species composition. Fragments S1, S2, M1 and M4 formed the cluster I and corresponded to the fragments with the lowest species density and richness (except M4) and with a MNFD between 200 and 400m. Fragments S3, S4 and S5 formed the second cluster formed by three small fragments highly isolated (300-600m) (Fig. 1). The cluster III was formed by the two large fragments, two medium ones (M2 and M3) and the eight ALL transects composing the calibration transect (MNFD 100-200m). Pas assez synthétique, tendance à répéter les résultats, le lecteur ne peut rien en retenir. The leaf litter ant communities can be assumed to be drawn from an identical local species assemblage because (1) the soils and forest types of the fragments (monte fuerte) are similar (Pujalte et al 1995); and (2) the distances between the forests did not translate into proportional distances in NMDS. Consequently, the decrease in species number and the variation in communities composition related to isolation degree of fragments most likely represent the effect of fragmentation rather than an eventual geographic effect as reported in literature (Majer 1983, Vasconcelos and Delabie 2000). Our NMDS analysis was reliable because of the very high intra-fragment similarity (M*). The distance between the eight transects of M* and M3 corresponded to the seasonal effect on ant species activity after a 10-month interval. This effect was relatively weak because the species density between M* and M3 was very close (Table1) and the composition highly similar. This is due to the close temperature conditions during the sampling periods. In the BDDFP experiment, several studies reported the isolation effects of the fragments on species composition for ants (Carvalho and Vasconcelos 1999, Brühl et al. 2003) and other taxa (Didham 1996). EDGE EFFECTS Ant species density did not vary as a function of distance to forest edge. This result is as it has been demonstrated in Amazonian forest fragmentation studies (Didham 1997). In contrast, ant species composition was influenced by edge effects. Half of the frequent species were forest species indifferent to the distance from the edge. Among the others species, we found four ubiquist species crossing over the sharp barrier between the forest and the grassland, six species preferentially collected at the edge, three in the centre and four species living only in the grassland. In spite of the sharp transition between the forest and the grassland, seven species were adapted to both habitats. Among them, we found generalists species as Pheidole sp.21 and Camponotus crassus or opportunist species as Wasmannia sp.01 or army-ant as Labidus preadator. Some factors, including light, microclimate (Kapos et al. 1997), and understorey productivity (Malcolm 1997) could account for changes in species composition at edges. Carvalho and Vasconcelos (1999) suggested that ant species composition differed in response to edge-related changes in litter depth. We previously demonstrated the close relation between the leaf litter weight and ant species distribution (Theunis et al. submitted). However, environmental factors measured along transects were not different between the edge and the center. The forest structure was identical from the edge to the centre of the fragment except in the L1 fragment where the bromeliads were concentrated in the middle of the transect. The species collected at the edge (and in the grassland or not) could be considered as ant edge-specialists. In contrast, the three species observed only in the centre was also collected in the small and medium fragment where the notion of edge and centre is indefinable. As a consequence, these species could not be considered as species centre-specialists. AKNOWLEDGMENTS FNRS FLIII Marius Delabie do nascimiento identification Isa carte APN, Guardaparques, sucunza, cornélio, instiuto tucuman REFERENCES Agosti, D. & Alonso, L.E. (2000a). The ALL protocol. A standard protocol for the collection of ground dwelling-ants. In: Agosti, D.; Majer, J. Alonso, L. & Schultz, T. The ALL protocol. A standard protocol for the collection of ground dwelling-ants. Smithsonian Institution Press: 204-206. Agosti, D., Majer, J., Alonso, L. and Schultz, T. (2000b) Ants- Standard methods for measuring and monitoring biodiversity. (Eds) Agosti, D.; Majer, J. Alonso, L.; Schultz, T. Smithsonian Institution Press : 280 p. Brühl, C.A., Eltz, T. and Linsenmair, K.E. (2003) Size does matter - effects of tropical rainforest fragmentation on the leaf litter ant community in Sabah, Malaysia. Biodiversity and Conservation 12:1371-1389. Carvalho, K.S. and Vasconcelos, H.L. (1999) Forest fragmentation in central Amazonia and its effects on litter-dwelling ants. Biological Conservation 91:151-157. Carvalho, K.S. and Vasconcelos, H.L. (1998) Forest fragmentation in central Amazonia and its effects on leaf-litter ants. Biotropica 30 (2 suppl.) Colwell, R.K. and Coddington, J.A. (1994) Estimating terrestrial biodiversity through extrapolation. Phil. Trans. R. Soc. Lond. B 345:101-11 Colwell, R.K. (1994-2004) EstimateS: Statistical estimation of species richness and shared species from samples. Version 7. Persistent URL <purl.oclc.org/estimates>. Delabie, J.H.C., Agosti, D. and do Nascimento, I.C. (2000) Litter ant communities of the Brazilian Atlantic rain forest region. D. Agosti, J. Majer, L.E. Alonso and T. Schultz Sampling Ground-dwelling ants: case studies from the worlds'rain forests. Curtin University, Australia, School of Environmental Biology, Bulletin no 18:1-17. Devillers, P. and Devillers-Terschuren, J. (1996a) A classification of South American habitats. IRSNB, Brussels, and Institute of Terrestrial Ecology, Huntinggdon: 419 p. Didham, R.K. (1996) Insects in fragmented forests: a functional approach. Trends in Ecology and Evolution 11: 255-260. Didham, R.K. (1997a) The influence of edge effects and forest fragmentation on leaf litter invertebrates in Central Amazonia. – In Laurence, W.F., Bierregaard, R.O. Jr (eds) Tropical forest remnants. Ecology, management, and conservation of fragmented communities. The University of Chicago Press : 55-70. Didham, R.K. (1997b) An overview of invertebrate responses to forest fragmentation. – In Chapman & Hall, London (eds). Forests and Insects Watt, A.D.; Stork, N.E.; Hunter, M.D.: 303-320. Dufrêne, M. and Legendre, P. (1997) Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecological Monographs 67: 345-366. Fisher, B.L. (1999) Improving inventory efficiency: a case study of leaf litter ant diversity in Madagascar. Ecological Applications 9: 714-731. Fisher, B.L. (1998) Ant diversity patterns along an elevational gradient in the Réserve Spéciale d'Anjanaharibe-Sud and on the Western Masoala Peninsula, Madagascar. Fieldiana Zoology (n.s.) 90: 39-67. Golden, D.M. and Crist, T.O. (2000) Experimental effects of habitat fragmentation on rove beetles and ants: patch area or edge? Oikos 90: 525-538. Gotelli, N.J. and Colwell, R.K. (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4:379-391. Gustafson, E.J. and Parker, G.R. (1994) Using an index of habitat patch proximity for landscape design. Landscape and Urban Planning 29: 117-130. Hammer, Øyvind, Harper, David A.T., and Paul D. Ryan, 2001. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4: 1-9. <http://folk.uio.no/ohammer/past/>. Laurance, W.F., Lovejoy, T.E., Vasconcelos, H.L., Brunia, E.M., Didham, R.K., Stouffer, P.C., Gascon, C., Bierregaard, R.O., Laurance, S.G. & Sampaio, E. (2002) Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conservation Biology 16: 605-618. Laurance, W.F. and Yensen, E. (1991) Predicting the impacts of edge effects in fragmented habitats. Biological Conservation 55: 77-92. Leponce, M., Theunis, L., Delabie, J.H.C. and Roisin, Y. (2004) Scale dependence of diversity measures in a leaf-litter ant assemblage. Ecography 27: 253-267. Levin S.A. (1992) The problem of pattern and scale in ecology. Ecology 73 (6): 1943-1967. Longino, J.T., Coddington, J. and Colwell, R.K. (2002) The ant fauna of a tropical rain forest: Estimating species richness three different ways. Ecology 83: 689-702. Majer, J. D., Day, J.E., Kabay, E.D. and Perriman, W.S. (1984) Recolonisation by ants in bauxite mines rehabilitated by a number of different methods. Journal of Applied Ecology 21: 355375. Magura, T., Ködöböcz, V. and Thóthmérész, B. (2001) Effects of habitat fragmentation on carabids in forest patches. Journal of Biogeography 28: 129-138. Majer, J.D., Delabie, J.H.C. and Mckenzie, N.L. (1997) Ant litter fauna of forest, forest edges and adjacent grassland in the Atlantic rain forest region of Bahia, Brazil. Insectes Sociaux 44:255266. Magurran, A.E. 1988. Ecological Diversity and its measurement, Chapman and Hall (Eds), Princeton University Press, New Jersey, 179 p. Melo, A. S., Pereira, R. A. S., Santos, A. J., Shepherd, G. J., Machado, G., Medeiros, H. F. and Sawaya, R. J. (2003) Comparing species richness among assemblages using sample units: why not use extrapolation methods to standardize different sample sizes? Oikos 101: 398-410. Minchin, P.R. (1987) An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio 69: 89-107. Patton, D.R. (1975) A diversity index for quantifying habitat edge. Wildlife Society of Bulletin 3: 171-173. Pfeifer et al. 1998 Pujalte, J.C., Reca, A.R., Balabusic, A., Canevari, P., Cusato, L. and Fleming, V.P. (1995) Anales de parques nacionales. Unidades Ecológicas del parque nacional Rio Pilcomayo. Administración de Parques Nacionales XVI: 1-185. Roisin Y. and Leponce M. (2004) Characterizing termite assemblages in fragmented forests: a test case in the Argentinian Chaco. Austral Ecology 29: 637-646. Simberloff, D. (1979) Rarefaction as a distribution-free method of expressing and estimating diversity. – In: Grassle, J.F., Patil, G.P., Smith, W. And Taillie, C. (eds), Ecological diversity in theory and practice. International Co-operative Publishing House, pp. 159-176. Soberon, J. and Llorente, J. (1993) The use of species accumulation functions for the prediction of species richness. Conservation Biology 7: 480-488. Stout, J. and Vandermeer, J. (1975) Comparison of species richness for stream-inhabiting insects in tropical and mid-latitude streams. American Naturalist 109: 263-280. Vasconcelos, H.L., Vilhena, J.M.S. and Caliri, G.J.A. (2000) Responses of ants to selective logging of a central Amazonian forest. Journal of Applied Ecology 37: 508-514. Vasconcelos, H.L. (1999) Effects of forest disturbance on the structure of ground-foraging ant communities in central Amazonia. Biodiversity and Conservation Kluwer Academic Publishers 8: 409-420. Vasconcelos, H.L. (1988) Distribution of Atta (Hymenoptera, Formicidae) in “terra-firme” rain forest of Central Amazonia: density, species composition and preliminary results on effects of forest fragmentation. Acta Amazônica 18: 309-315. Vasconcelos, H.L., Carvalho, K.S. and Delabie J.H.C. (1998). Ecology and conservation of a fragmented forest: some perspectives based on studies of ground ant communities. - In the Ecology and Conservation of a Fragmented Forest: lessons from Amazonia. Bierregaard Jr, R.O., Gascon, C., Lovejoy, T.E. and Santos, A.A. (eds). Yale University Press. Vasconcelos, H.L. and Delabie J.H.C. (1998) A study of forest fragmentation near Manaus, Brazil. – In Measuring and monitoring biodiversity: standard methods for ground-dwelling ants. Agosti, D., Majer, J., Alonso, Schultz, T. and L. Tennant (Eds). Washington, DC: Smithsonian Institution Press. Williams-Linera, G. (1990) Vegetation structure and environmental conditions of forest edges in Panama. Journal of Ecology 78: 356-373. Fig. 1: Map of the Rio Pilcomayo National Park, Argentina, showing the location of the eleven forest fragments where the transects were performed. Fragments belonged to three size categories: small (S, < 4ha, n=5), medium (M, between 15 and 25ha, n=4) and large (L= 250ha, n=2). (white zones= grassland, grey= forest fragments). Fig. 2: Comparison of species richness in small (S, n=5), medium (M+M*, n=12) and large (L, n=2) fragments. Species richness was measured with A.L.L. Winkler transects and was standardized for 232 occurrences by Melo’s method (Melo et al. 2003). Figure 3: Non-metric multidimensional scaling (two dimensions, stress= 0.1122) based on the standardized Bray-Curtis index of similarity (calculated on ant species frequencies) between fragments (S= small, M= medium and L= large). M* represents the intra-fragment variability of this measure. Clusters were determined by a UPGMA analysis (similarity= 0.7). Ant composition split into three clusters at 50 and 60 % of similarity (UPGMA, cluster analysis). Axe 1 – Nearest neighbour, r= -0.46* Axe 2- Proximity Index, r= -0.48* Fig. 4: Distribution of species from the surrounding grassland towards the centre of large forest fragments. (A) Average species density along the 3 parallel transects in L1 (n= 115 quadrats devoid of Bromeliaceae, Winkler extraction); (B) number of species collected by pitfall traps along the central transect performed in L1; (C) number of species collected by pitfall traps along the central transect performed in L2. Table 2: Significativity of the difference between the species richness values (by bootstrapping) between each fragment (S= small, M= medium and L= large). S1 S2 S3 S4 S5 M1 M2 M3 M4 L1 L2 S1 1 S2 ns 1 S3 ** ** 1 S4 *** ** ns 1 S5 ns ns ns * 1 M1 * * ns ns ns 1 M2 ** * ns ns ns ns 1 M3 *** *** * ns ** ** ** 1 M4 ** ** ns ns ns ns ns ns 1 L1 *** *** ns ns * * * ns ns 1 L2 ** ** ns ns ns ns ns ns ns ns 1 Table 3: Effect of fragment size, shape and isolation on ant species richness and density. Pearson’s correlation coefficients and degree of significance p (ns= no significative, *< 0.05, **< 0.01 and ***< 0.001) between the mean species density (number of species/m²), standardized species richness (for 232 occurrences) and the fragmentation indices of each forest fragment. Standardized species p Species density richness Area (log) 0.60 * 0.45 Perimeter (log) 0.66 * 0.46 Shape Index 0.81 ** 0.37 log P/ log A -0.21 ns -0.31 Proximity Index 0.56 0.07 0.27 Nearest Fragment -0.15 ns -0.31 Mean Nearest Fragment Distance -0.19 ns -0.41 Fragmentation Index p ns ns ns ns ns ns ns Table 4: Habitat preferences of species frequent either in the forest or in the grassland or in both. Ants were collected in large forest fragments (L1 and L2) by both collection methods (Winkler and pitfall traps) and in the grassland only by pitfall traps. In brackets we indicated the distance from the edge to which the species was collected (at edge) or the distance from which the species was collected (at the centre). A Mann-Whitney U test allowed to compare the abundance of species (log10 (x+1)-transformed) near the edge (< 250m) or near the centre (>250m). A significantly greater abundance of species near the edge or near the centre was indicated by “XX” in the corresponding column. Forest Species Centre Edge Species abundance comparison between the edge and the centre Grassland Crematogaster sp.18 X (50m) X Paratrechina pubens X (100m) X Pheidole aberrans Hypoponera sp.08 Paratrechina sp.01 Solenopsis sp.14 Solenopsis sp.16 Brachymyrmex sp.05 Paratrechina sp.02 X (200m) X X X X X X (150m) X (100m) X (100m) X X X XX XX XX * * 0.08 Camponotus crassus Labidus praedator Pheidole sp.21 X X X X X X X X X X X X X X X X X X X X X X X X X X X X NS NS NS NS NS NS NS NS NS NS NS NS NS NS X X X Wasmannia sp.01 X X NS X Pyramica denticulata Carebarella bicolor Crematogaster sp.11 Solenopsis Sp.18 Acromyrmex hispidus fallax Crematogaster sp.02 Crematogaster sp.17 Gnamptogenys striatula Octostruma rugifera Pachycondyla harpax Pheidole flavens Pheidole nubila Solenopsis sp.01 Solenopsis sp.02 Solenopsis Sp.17 Table 1: Summary of fragmentation indices, sampling informations and diversity results for each fragment Plog= perimeter (log-transformed), SI= Shape Index, PI= Proximity index, NF= nearest fragment, MNFD= Mean nearest fragment distance, FRAGMENTATION INDICES vs. ANT ASSEMBLAGE Sampling informations fragmentation indices Area (ha) Plog (m) SI log P/ log A PI NF (m) MNFD (m) Date T° MAX Transects (N, samples) Diversity results Sample Size Sampling method Forest Grassland Bromeliads samples S obs I Inventory completeness S extrapolated (I= 232) S density S1 1.6 2.85 1.56 0.676 1.45 20 218 29.4 1*20 20 20 58 asymptotic 28.6 2.9 (± 1.8) S2 1.1 2.80 1.66 0.689 0.79 100 330 36.6 1*20 20 23 78 logarithmic 34.2 3.25 (± 1.5) S3 3.5 3.06 1.74 0.674 0.02 30 353 34.7 1*20 20 35 125 asymptotic 39.6 6.25 (± 2.9) S4 2.2 2.90 1.52 0.669 0.22 20 297 35.8 1*20 20 40 157 asymptotic 43.1 7.85( ± 3.3) S5 3.0 2.95 1.44 0.659 0.03 580 610 20.8 1*20 20 25 71 logarithmic 39.0 3.6 (± 3.2) M1 20.5 3.48 1.88 0.655 0.71 20 287 35.7 1*20 20 28 82 asymptotic 37.0 4.1 (± 2.9) M2 27.9 3.54 1.85 0.650 0.06 60 180 20.5 1*20 20 34 143 logarithmic 40.0 7.2 (± 3.0) M3 16.1 3.56 2.55 0.684 11.93 20 152 21.8 1*20 46 159 logarithmic 53.0 8.0 (± 3.6) M4 28.8 3.65 2.35 0.668 0.05 40 356 33.9 1*20 34 90 logarithmic 50.2 4.5 (± 2.4) 44 178 logarithmic 48.6 5.9 (± 3.2) 44 232 logarithmic 44.0 7.7 (± 3.7) logarithmic 46.6 (± 2.2) 7.3 (± 3.7) 20 Winkler 20 All quadrats 0 L1 256.2 4.09 2.19 0.639 13.92 10 199 38.5 3*50 30 patches of bromeliads and after 200m from the edge L2 254.4 4.04 1.94 0.631 0.09 120 321 38.8 1*50 30 After 200m from the edge M*1-8 16.1 3.56 2.55 0.684 11.93 20 152 30.2 8*20 160 all quadrats Winkler EDGE EFFECTS L1 3*50 L1 1*55 L2 1*55 Winkler 114 0 Bromeliads discarded 65 3.6 (± 2.5) INTROGRESSION Pitfall 50 5 5 (0 - 11) 50 5 3 (0 - 9)