Ecosystem services
advertisement

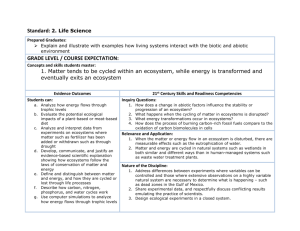
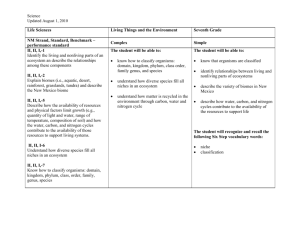
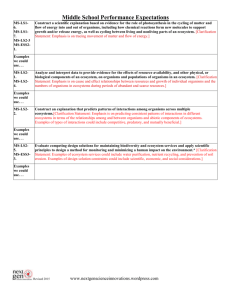
Read: Ecosystem Services Ecosystems Ecosystem: all living things in a particular place and the nonliving (abiotic) environment in which they live o All components of an ecosystem interact with one another o Components of an ecosystem are highly interconnected o Different ecosystems are connected Ecosystems defined at different levels Salt marsh (where ocean meets lakes/rivers) [Mississippi River, Gulf of Mexico, land… lots of interactions] Energy and matter in ecosystems Ecosystems provide services that are essential to support all life on earth o Capture and transfer of energy Energy flows through ecosystem Earth is an open energy system o Storage and transfer of matter Matter and elements cycle through ecosystems The right amount of matter at the right time to support Earth is a closed system with respect to matter (no matter is ever created or destroyed, only recycled through the system) Ecosystems energy The earth is an open system with regard to energy o Constant energy from the sun o Very little energy from the sun is captured for use o Emits thermal energy into space Figure 57.10 Capture of matter and energy Autotrophs: synthesize organic compounds from inorganic molecules o Auto = self; troph = feeding o Photosynthetic: energy from sunlight to make organic compounds (sugars) o Autolithortrophs: oxidize inorganic compounds (H2S, S, Fe, H2) to obtain energy to make organic compounds Transfer of matter and energy Heterotrophs: cannot synthesize organic compounds Live by taking organic compounds and energy from other organisms o Primary consumers: eat producers o Secondary consumers: eat primary consumers Trophic level: an energy/matter transfer level in a food chain Trophic level energy transfer Figure 57.8 How much energy is available to producers? Consumers? 2nd Consumers? Remember the 2nd law! Nature is always increasing entropy, always losing some energy. About 10% available as we move up trophic levels Energy loss in organisms Figure 57.9 Most acquired energy is lost as heat Living organisms can not transfer heat energy Only energy stored in chemical bonds (biomass) can move up to the next trophic levels Ecological Pyramids Figure 57.12, Figure 57.13 Matter in an ecosystem All organisms are made of 6 primary atoms: C H O N P S o Plus: Na Cl K Ca Mg Fe (smaller amounts) Limited amount of C H O N P S Matter cycles through biotic and abiotic parts of an ecosystem o Proper cycling required for life o Disruption of cycling decreases capacity of the earth to support life Cycling of CHONPS We’ll ignore small and trace elements Concentrate on C H O N P S plus H2O o Each has reservoir o At any time most of an element is in its reservoir Reservoir of element Return Incorporation Organisms Reservoirs Can be: o Air: atmosphere o Water: ocean, lakes, streams o Sediment: soil, rocks, seabed Differ vastly in how accessible they are Role of decomposers important: o Make C H O N P S available from dead organisms, leaf litter and other waste etc. o Recycling Two major types of reservoir Reservoir is water or atmosphere o Carbon: CO2 of atmosphere (primary) o Nitrogen: N2 of atmosphere (80%) o Hydrogen: H2O (primary) o Oxygen: H2O (primary), CO2 Reservoir is sediment o Phosphorous: PO43- seabed, soil, rocks o Sulfur: sediment (primary), air The water cycle Water circulates from earth’s surface to the atmosphere Relies on evaporation & precipitation Evaporation o Occurs directly from bodies of water. (heat must be present) o Terrestrial ecosystems rely on plants for evaporation (transpiration – stomata on leaves to get CO2) o Purifies the water Move H2O from soil to atmosphere Brings about precipitation Figure 57.2 Disruption of the water cycle Deforestation (large scale clear cutting) o Permanently change rainfall patterns Development – malls, schools, parking lots, factories, etc. o Increase water runoff and fresh water loss o Pollution (ex: New York City) o Prevents evaporation cooling: urban heat island Affects other cycle: o Loss of organic nutrients o Alters retention of bio-available forms Read: Carbon cycle Carbon is cycled through the biosphere through photosynthesis and respiration o Photosynthesis: CO2 + H2O -> organic sugars (synthesis) o Respiration: Organic sugars -> CO2 + H2O (degredation) Carbon cycling inputs and outputs can be and are unbalanced Figure 57.1 Carbon, Hydrogen, & oxygen: all interrelated Connects plants to everything else Connects photosynthesis & respiration The nitrogen cycle No shortage of N o 80% of air is N2 but N2 is very unreactive o not usable by most organisms o scarcity of useable nitrogen o Must convert to usable form Two sources of useable nitrogen: o Recycling o Fixation Recycling of organic nitrogen Occurs through microorganisms Are major source of useable nitrogen o Decomposers reclaim usable N from dead organisms and waste products o Waste products (ammonium (NH4+) and urea are converted to ammonia (NH3) o Plants absorb NH3 – convert to nitrate (NO3-) o Animals get bio available N (NH3 and NO3-) from plants Nitrogen fixation: in nature Bacteria/plants Humans (industrial process) (not in your body) Bacterial Fixation: o Symbiotic association with legumes, clover, alfalfa, and peanuts o Converts atmospheric N2 into ammonia o Plant provides energy, bacteria provide N Endergonic: requires 16 ATP o Nitrifers convert some of NH3 to NO2- and NO3Nitrogen fixation: the hard way Haber process o Reaction of N and H using an iron catalyst o Produces ammonia (NH3) Energetically expensive o Use energy from fossil fuels and hydrocarbons o 30% agricultural energy use – fertilizer production Fertilizer: human additions to soil o Sustains 1/3 of earth’s population o Also creates excessive runoff Figure 57.4 Nitrogen runoff: effects Figure 57.7 Eutrophication results from over abundance of nutrients in the water o Fertilizer run off is a main cause o Algal bloom results in wild O2 fluctuations o Toxic byproducts from some algae Dead zone: hypoxic (low oxygen) areas o No marine life due to low oxygen At least 170 dead zones around the world o Range in size: 1 miles2 to over 6,000 miles2 Phosphorous P does not cycle through atmosphere Free P (PO43-) is bioavaliable in soil/sediment Uptake by plants – enters food chain Returns to soil/sediment by decomposition P can be a limiting nutrient o Precipitates with Ca2+ and Mg2+ and forms nutrients o Only available now with erosion/uphearal – slow! o PO43- easily washed away o Added to soil as fertilizer/pesticides o 3.5 million tons/year enter ocean as waste Figure 57.5 Sulfur Sulfur not a limiting nutrient o Organisms require only small amount Sulfur comes to living systems from airborne particles o From volcanic activity o Reaches living systems with rain Also found in coal and oil deposits o High sulfur coal is an environmental problem o Burning produces acid rain Ecosystem services-1 Natural ecosystems provide essential nutrients to living organisms What else do they do? o Maintain biodiversity Nature’s pharmacy: 9 of the top 10 drugs are natural plant products 118 of the top 150 drugs come from nature Food source for humans (fisheries) o Detoxify and decompose wastes o Purify air and water Ecosystem services-2 What else do they do? o Mitigate droughts and floods o MRGO Moderate weather extremes o Wind and evaporative cooling from oceans resists global warming Maintain wind and weather patterns Pollination o 1/3 of food supply in USA is naturally pollinated o 100,000 animals provide free pollination The value of ecosystem services Aesthetic value The cost of replacing ecosystem services o Services poorly understood, undiscovered o Duplicating ecosystem services o Ecosystem services valuation and the market o Policies encourage conversation of ecosystems to uses that are traded at market