Progesterone ELISA Test Kit
advertisement

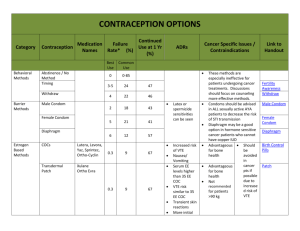
Progesterone Page1 Atlas Link 12720 Dogwood Hills Lane, Fairfax, VA 22033 USA Phone: (703) 266-5667, FAX: (703) 266-5664 http://www.atlaslink-inc.com, info@atlaslink-inc.com PROGESTERONE ELISA For in vitro diagnostic use. Catalog No. 2077 Enzyme immunoassay for the quantitative determination of Progesterone in human serum or plasma Store at 2 to 8 °C EXPLANATION OF THE TEST Progesterone (4-pregnene-3, 20-dione; MW 318) is a C21 steroid. In the nonpregnant female it is secreted in a cyclic manner by the ovaries. Smaller quantities are also secreted by the adrenal cortex of both men and women ( 1). Approximately 97% of progesterone in plasma is protein bound. The free progesterone is the physiologically active form of the hormone. Progesterone is metabolized to pregnanediol and is excreted as a glucuronide conjugate (2). Progesterone concentrations are low in the follicular phase of the menstrual cycle. There is a slight rise at the time of the gonadotrophin surge and then a dramatic increase reaching a maximum level 5 to 6 days after ovulation as progesterone is secreted from the corpus luteum. If fertilization does not occur the corpus luteum regresses and progesterone concentrations fall towards the end of the cycle (3). However, if implantation occurs the corpus luteum continues to secrete increasing amounts of progesterone until the 10th to 12th week of pregnancy when the placenta takes over as the principal site of progesterone production. In the investigation of infertility, assays of serum progesterone concentrations are used to confirm whether ovulation has occurred either naturally or as a result of treatment with other ovarian stimulants. Recurrent or threatened abortion in early pregnancy due to corpus luteum dysfunction can be diagnosed by serial progesterone measurements, which will be lower than normal. PRINCIPLE OF THE TEST The Atlas Link Progesterone EIA is an enzyme linked immunoassay incorporating an anti-progesterone monoclonal antibody (Antibody Reagent) and an anti-murine IgG polyclonal antibody bound to microwells. It is a one-step ("competitive" ) method utilizing a progesterone-horseradish peroxidase conjugate (progesterone-HRP; Conjugate Reagent) to produce the signal generated. The assay can be used to measure progesterone from 0 to 1 00 nmol/L. The incorporation of a synthetic ligand, specific for plasma binding proteins, eliminates endogenous binding of progesterone and allows the direct assay of progesterone in serum without prior extraction or chromatographic separation. The highly specific antibody used in the assay shows no cross-reaction with the specific ligand. During incubation of sample, Conjugate Reagent and Antibody Reagent in the microwell, complexes are formed between monoclonal antibody and sample progesterone antigen or progesterone-HRP. This occurs as the antibody itself is captured by the microwell-bound Atlas Link, 12720 Dogwood Hills Lane, Fairfax, VA 22033 USA Phone: (703) 266-5667, FAX: (703) 266-5664 http://www.atlaslink-inc.com, info@atlaslink-inc.com Progesterone Page2 secondary antibody. The microwells are washed to remove material not captured. Then Substrate Solution, containing TMB and peroxide, is added. These components react with the peroxidase to produce color (blue) in inverse proportion to the amount of antigen in the sample. From photometric absorbance readings a standard curve is constructed and the progesterone in patient samples can be quantitated. REAGENTS REAGENT PREPARATION Reagents are sufficient for 96 wells. Allow reagents and samples to reach room temperature (18 to 25 °C) before use. 1. Antibody Coated Wells 96 wells Contents: Microwells coated with goat anti-murine IgG polyclonal antibody. Preparation: Ready to use. Storage: Keep the microwell plate in a sealed bag with desiccant and minimize exposure to damp air. 2. Reference Standard Set 1 set of 6 vials Contents: Nominal values are 0,1, 3, 10, 30 &100 nmol/L progesterone in processed human serum with 0.05% thimerosal. Actual progesterone concentrations, determined lot-to-lot, are stated on each standard vial label. Preparation: Reconstitute the lyophilized standards by accurately pipetting 0.5 mL (2.0 mL for the zero standard) deionized water into each of the vials. Cap and mix well by gently swirling or inversion. Allow to reach room temperature before use. Stability: Reconstituted standards are stable for up to four weeks at 2 to 8 ° C. 3. Conjugate Reagent 5 mL Contents: Progesterone conjugated to horseradish peroxidase in a stabilizing buffered solution containing a violet dye. Contains 0.2% (w/v) Bronidox L and 0.01% (w/v) thimerosal as preservatives. Preparation: Ready to use. 4. Antibody Reagent 5 mL Contents: Murine monoclonal anti-progesterone antibody in a phosphate-buffered saline solution containing purified animal serum proteins and blue dye. Contains 0.2% (w/v) Bronidox L and 0.01% (w/v) thimerosal as preservatives. Preparation: Ready to use. 5. Wash Concentrate Contents: preservative. 50 mL Concentrated (15X) wash solution with 0.15 % thimerosal as a Preparation: Prepare Wash Solution by diluting the Wash Concentrate 1:15 with deionized or distilled water. Mix well. Atlas Link, 12720 Dogwood Hills Lane, Fairfax, VA 22033 USA Phone: (703) 266-5667, FAX: (703) 266-5664 http://www.atlaslink-inc.com, info@atlaslink-inc.com Progesterone Page3 Concentrate 15 mL 30 mL 50 mL Storage: Dilute to 225 mL 450 mL 750 mL Enough for 32 wells 64 wells 96 wells Diluted Wash Solution may be stored for up to twelve weeks at 20 to 25 ° 6. Substrate Solution C. 10 mL Contents: 3,3',5,5'-tetramethylbenzidine (TMB) and hydrogen peroxide in a stabilizing solution. Preparation: Ready to use. 7. Stop Solution. 10 mL Contents: 2N Hydrochloric acid. Preparation: Ready to use. Warnings and Precautions for Users 1. 2. 3. 4. 5. 6. 7. 8. CAUTION: Human source materials: Treat as potentially infectious. Each serum donor unit used in the manufacture of this product is tested by an FDA approved method and found non-reactive for the presence of HBsAg and antibody to HIV-1. Because no known test method can offer complete assurance that Hepatitis B Virus, Human Immunodeficiency Virus (HIV-1), or other infectious agents are absent, all human blood products, including human source material, should be handled in accordance with good laboratory practices using appropriate precautions (e.g., Centers for Disease Control/National Institutes of Health Manual, "Biosafety in Microbiological and Biomedical laboratories", 1988). Avoid contact with the Stop Solution. It containhydrocloric acid which may cause skin irritation and burns. Some of the reagents in this kit contain thimerosal and Bronidox L as preservatives. These are toxic and therefore all reagents should be handled carefully to avoid ingestion or skin contact. Do not use reagents after the expiration date. Do not mix or use components from kits with different lot numbers. Replace caps on reagents immediately. Do not switch caps. Do not pipette reagents by mouth. For in vitro diagnostic use only. STORAGE CONDITIONS Store the kit at 2 to 8 C upon receipt and when it is not in use until the expiration date shown on the kit box label. INSTRUMENTATION A microwell reader with a bandwidth of 10 nm or less and an optical density range of 0 to 2 A or greater at 450 nm wavelength (A450) is acceptable. An orbital motion microplate shaker is necessary. SPECIMEN COLLECTION AND PREPARATION 1. 2. 3. 4. No special patient preparation is required. Test specimens can be either serum or plasma collected in a manner appropriate for laboratory testing. Serum is preferred although the anticoagulants heparin or EDTA can be employed without sacrificing accuracy. Avoid grossly hemolytic, lipemic and turbid samples. Specimens can be stored at 2-8 C for up to 48 hours. Specimens held for longer should be stored at or below -20 C. Specimens should not be frozen and thawed repeatedly. Thawed specimens should be checked for flocculent matter and mixed by gentle inversion just prior to testing. Turbid samples or samples containing particulate matter should be centrifuged prior to use. TEST PROCEDURE Materials Provided With The Test Kit Atlas Link, 12720 Dogwood Hills Lane, Fairfax, VA 22033 USA Phone: (703) 266-5667, FAX: (703) 266-5664 http://www.atlaslink-inc.com, info@atlaslink-inc.com Progesterone Page4 1. 2. 3. 4. 5. 6. 7. Antibody Coated Microwells, 96 wells Reference Standard Set, 0.5 mL per vial Enzyme Conjugate Reagent, 5 mL Antibody Reagent, 5 mL Wash Concentrate, 50 mL Substrate Solution, 10 mL Stop Solution, 10 mL Materials Required But Not Provided 1. Distilled or deionized water 2. Precision pipette: 50 L, 500 L 3. Repeating or 8-channel pipette: 50 L, 100 L 4. Disposable pipette tips 5. Measuring cylinder (1L) for preparing Wash Solution 6. Microwell plate reader 7. Absorbent paper 8. Graph paper 9. Control sera (recommended) 10. Plate shaker Procedural Notes 1. 2. 3. 4. 5. 6. Bring all reagents and specimens to room temperature (20 to 25 C) and mix by gentle inversion prior to use. A standard curve should be run with each assay. Ensure that the standard values for each kit match those used for data reduction. Microwells may be used only once. Avoid touching the bottom of the wells to minimize optical interference. Before reading the plate wipe the underside of the wells with lint-free tissue. All assay steps should be performed without interruption but if the wells cannot be filled with Substrate Solution immediately after washing then the plate may be left upside down on absorbent lint-free tissue for a maximum of 15 minutes. Reagents are matched in each kit and therefore reagents from different lot numbers should not be mixed. The plate reader and all pipettes used should be calibrated correctly before use. It is recommended that a pipette tip be attached to the repeating pipette unit to increase the precision of the Antibody Reagent and Conjugate Reagent additions. These are critical dispensing steps for any immunoassay of this type. Washing The efficiency of the wash step is vital for good precision. Plates can be washed manually or by using an automatic plate washer. Aspirate the liquid and rinse each well four times with 250 L Wash Solution. Avoid overflows from one well to another. After the final wash, the plate should be inverted and tapped firmly on absorbent lint-free tissue to remove the last traces of wash buffer. Ensure that no bubbles remain in the wells before proceeding to the next step. Equipment should be kept clean and primed with Wash Solution prior to use. Wash Solution should be stored in clean containers to prevent contamination with substances which could interfere with the enzyme. The washer should not be left standing with Wash Solution for long periods of time. At the end of each day the washer should be rinsed with distilled water. Regular cleaning according to the washer instruction manual should be carried out. Conjugate and Substrate Contamination of these reagents will lead to poor performance. Use dedicated dispensers for these reagents. Avoid contact with metallic surfaces since these can interfere with the Substrate. Assay Procedure 1. 2. 3. 4. 5. Secure the desired number of coated wells in the holder. Dispense 50 L of standards, samples, and controls into appropriate wells. Add 50 L of Enzyme Conjugate Reagent (violet) into each well. Add 50 L of Antibody Reagent (blue) into each well. Cover plate with lid and incubate at room temperature for 60 minutes on a microwell plate shaker. Atlas Link, 12720 Dogwood Hills Lane, Fairfax, VA 22033 USA Phone: (703) 266-5667, FAX: (703) 266-5664 http://www.atlaslink-inc.com, info@atlaslink-inc.com Progesterone Page5 After incubation wash the plate. Aspirate the liquid and rinse each well four times with 250 L Wash Solution. After the final wash, invert the plate and tap firmly on absorbent tissue to remove any remaining Wash Solution. Ensure that no bubbles remain in the wells before proceeding to the next step. 7. Add 100 L of Substrate Solution into each well. This step should be carried out smoothly and without interruption. Timing of the incubation step is measured from the addition of substrate solution to the first well. 8. Cover plate and incubate at room temperature for 5 minutes. 9. Stop reaction by adding 50 L of Stop Solution to wells in the same sequence that the Substrate Solution was added. 10. Place microwell plate into the microplate reader ensuring that the plate is properly located. 11. Read the absorbance at 450 nm (A450). 6. RESULTS Calculation of Results 1. 2. 3. 4. 5. Calculate the mean absorbance values (A450) for the duplicate Reference Standards, controls, and patient samples. Using log-linear graph paper, construct a standard curve by plotting the mean absorbance obtained for each Reference Standard against its concentration in nmol/L, with absorbance on the vertical (y) axis and concentration on the horizontal (x) axis. Using the mean absorbance value for each sample, determine the corresponding concentration of progesterone in nmol/L from the standard curve. Computer data reduction can also be employed. Any diluted samples must be converted by the appropriate dilution factor. Progesterone values obtained are in the SI units, nmol/L. Conversion to ng/mL may be accomplished using the following equation: Progesterone (ng/mL) = Progesterone (nmol/L) x 0.314 Interpretation of Results 1. Results of a typical standard run are shown below: Progesterone (nmol/L) 0 1.00 3.01 10.0 30.0 100 1 2.827 2.209 1.686 0.846 0.358 0.200 A 450 nm 2 2.798 2.250 1.759 0.887 0.376 0.200 Mean 2.815 2.230 1.723 0.867 0.367 0.200 2. Standard Curve NOTE: This standard curve is for the purpose of illustration only, and should not be used to calculate unknowns. Each laboratory must provide its own data and standard curve. EXPECTED VALUES Atlas Link, 12720 Dogwood Hills Lane, Fairfax, VA 22033 USA Phone: (703) 266-5667, FAX: (703) 266-5664 http://www.atlaslink-inc.com, info@atlaslink-inc.com Progesterone Page6 It is recommended that each laboratory establish its own reference ranges for progesterone based on a representative sample population. The following normal reference ranges, calculated as 95% confidence intervals, were obtained by assaying serum samples from apparently healthy individuals and are given as a guide only: Sample Grouping Adult Male Adult Female Follicular Phase Luteal Phase Postmenopausal n 68 36 40 11 Reference Range (nmol/L) < 4.5 (2.2) 0.7 - 3.5 (2.1) >>5* < 1.6 (0.7) * generally < 60 nmol/L; ( ) bracketed numbers are means; n is the number of samples assayed During pregnancy the following values generally may be expected (7): 1 st trimester < 100 nmol/L 2nd trimester 100 - 300 nmol/L 3rd trimester > 300 nmol/L QUALITY CONTROL Good laboratory practice requires that low, medium, and high controls are run with each calibration curve. A statistically significant number of controls should be assayed to establish mean values and acceptable ranges to assure proper performance. Controls containing azide should not be used. LIMITATIONS OF PROCEDURE 1. Reliable and reproducible results will be obtained when the assay procedure is carried out with a complete understanding of the package insert instructions and with adherence to good laboratory practice. 2. The wash procedure is critical. Insufficient washing will result in poor precision and falsely elevated absorbance readings. PERFORMANCE CHARACTERISTICS 1. Precision a. Intra-assay Precision Intra-assay precision (%CV) was evaluated on 3 samples measured 16 times in the same assay. b. SampleMean 2SD (nmol/L) %CV 1 3.35 0.34 5.1 2 18.5 0.91 2.5 3 41.4 2.62 3.2 Inter-assay Precision Inter-assay precision (%CV) was evaluated on 3 samples measured in duplicate in 40 different assays. SampleMean 2SD (nmol/L) %CV 1 3.40 0.59 8.7 2 19.9 2.87 7.2 3 46.4 6.79 7.3 2. Sensitivity The sensitivity of the assay is typically less than 0.25 nmol/L. The sensitivity is defined as that concentration of progesterone which corresponds to the dose response variable (mOD/min or OD) that is two standard deviations less than the mean dose response variable of 20 replicate determinations of the zero calibrator run in three different assays. 3. Interference Atlas Link, 12720 Dogwood Hills Lane, Fairfax, VA 22033 USA Phone: (703) 266-5667, FAX: (703) 266-5664 http://www.atlaslink-inc.com, info@atlaslink-inc.com Progesterone Page7 No interference with progesterone recovery was observed for concentrations of hemoglobin up to 250 mg/dL, bilirubin up to 10 mg/dL, and triglycerides up to 970 mg/dL. 4. Specificity Specificity of the monoclonal antibody used in this kit was assessed by calculating the percentage crossreactivity of various compounds. Compound Progesterone 17-OH-Progesterone Pregnenolone Deoxycorticosterone Androstenediol Cholesterol Corticosterone Cortisol 11 -Deoxycortisol 17-Estradiol 17-Estradiol Estriol Estrone Pregnanolone 20-OH-Progesterone 20-OH-Progesterone Testosterone % Cross-reactivity 100 1.8 1.1 0.47 <0.2 <0.2 <0.2 <0:2 <0.2 <0.2 <0.2 <0.2 <0.2 <0.2 <0.2 <0.2 <0.2 Atlas Link, 12720 Dogwood Hills Lane, Fairfax, VA 22033 USA Phone: (703) 266-5667, FAX: (703) 266-5664 http://www.atlaslink-inc.com, info@atlaslink-inc.com Progesterone Page8 REFERENCES 1. 2. 3. 4. 5. 6. 7. Abraham GE. The application of natural steroid immunoassay to gynecologic endocrinology. In: Abraham GE (ed). Radioassay Systems in Clinical Endocrinology. New York: Marcel Dekker; 1981: 475-529. Gautreay JP, de Brux J, Trajchner G, Robel P, Mouren M. Clinical investigation of the menstrual cycle; clinical endometrial and endocrine aspects of luteal defect. Fertility and Sterility 1981; 35: 296-303. Ismail AAA. Biochemical Investigations in Endocrinology. Methods and Interpretations. London: Academic Press; 1981. McGinley R, Casey JH. Analysis of progesterone in unextracted serum: a method using Danazol (17 pregn-4-en-20-ynol (2,3-d) isoxazol-17-ol) a blocker of steroid binding to proteins. Steroids 1979; 33: 127138. Ratliffe WA, Corrie JET, Dalzeil AH, MacPherson JS. A general approach to the direct assay of progesterone in unextracted serum using a heterologous bridge system and a 125I-radioligand. Clin Chem 1982; 28: 1314-18. Jacobson RH, Downing DR, Lynch TJ. Computer assisted enzyme immunoassays and simplified immunofluorescence assays. Applications for the diagnostic laboratory and veterinarian's office. J Am Vet Med Assoc 1982; 181 (10):1166-68. Casey ML, MacDonald PC, Simpson ER. In: Wilson JD, Foster DW (eds) Williams Textbook of Endocrinology. 8th ed. Philadelphia: WB Saunders Co; 977-991. TECHNICAL CONSULTATION Call or Write: 23961 CRAFTSMAN ROAD SUITE E/F CALABASAS, CA 91302 U.S.A.. T F E A L X : (818) 591-3030 : (818) 591-8383 Rev. A - 1997 Atlas Link, 12720 Dogwood Hills Lane, Fairfax, VA 22033 USA Phone: (703) 266-5667, FAX: (703) 266-5664 http://www.atlaslink-inc.com, info@atlaslink-inc.com