Anesthesia and Analgesia for Research Animals

Hazardous and radioactive waste disposal:
carcass, bedding, cages and PPE
Date Issued: June 23, 1999
Date Revised: April 14, 2009 (
D. Larsen, ed.)
Comparative Medicine Resources
Purpose:
To describe procedures for safe disposal of carcasses, bedding, cages and personal protection equipment for rodent studies involving:
Pathogenic microorganisms
Radioisotopes
Toxins and carcinogens.
[
For chemicals that require special disposal procedures see developed specific SOPs (i.e., botulinum toxin, STZ, diphtheria toxin, etc.])
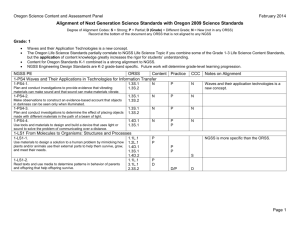
I. Pathogenic microorganism (ABSL2)
A. Carcasses
1. When animals are found dead (FD) or euthanized by the vivarium staff, the staff member will label an appropriately sealed red plastic bag with the investigator’s name, protocol number, study number, how many animals, if they were FD or euthanized, the person’s initials and the date.
2. On the right side of the cage card under Investig ator’s notes the technician will write the number of animals, if it (they) was (were) FD or euthanized, the date and their initials. The number of animals on the cage card will be changed to reflect the actual number in the cage and the number of animals removed will be put on the correct census sheet.
3. The animal carcass(es) is/are sealed in red plastic biohazard autoclave bags, taped loosely with autoclave tape and left in the animal room and are autoclaved daily .
[See Cagewash SOP for biological monitoring of autoclaves]
4. The lab contact person listed on the cage card is contacted by e-mail of the death or euthanasia. The vivarium supervisor, Chief Clinical Veterinarian and Associate
Director are copied on the e-mail.
5. Carcasses and cages shall be autoclaved minimally for 25 minutes sterilization time at 250F on the liquid setting with no drying time.
6. Once autoclaving is complete the bag (s) is allowed to cool, is placed in a black plastic bag and placed in the carcass freezer.
7. When the one of the freezers is close to full the supervisor or designee will call carcass management company for a pick up. Normally the pick up will be scheduled for the following business day.
8. Investigator staff finding dead or euthanizing animals will place them in the appropriate black bag and leave it in the housing room and the animal caretaker will remove it daily and autoclave the carcass in the bag.
1
9. Investigator staff must also change the information on the cage card and the census sheet in each animal housing room
B. Bedding
Bedding from studies with BSL-2 agents must not be dumped unless it has been decontaminated. Decontamination may take place in one of two ways by autoclaving of cages with dirty bedding.
C. Cages
1. Cages must be bagged in an autoclave bag labeled with the biohazard symbol.
2. Carcasses and cages shall be autoclaved minimally for 25 minutes sterilization time at 250F on the liquid setting with no drying time.
3. After autoclaving, dump the bedding into regulated medical waste containers.
4. Cages can then be cleaned using a standard cage-washing cycle.
D. Personal Protective Equipment (PPE) and other potentially contaminated items
PPE, sharps containers, and other potentially contaminated items must be discarded into the red bag for medical waste disposal.
II. Radioisotopes
A. Carcass disposal
1. The research laboratory makes arrangements with ORSS as to when and how
the animal will be dispose. ORSS office hours 9 am to 4:30pm Mon-Fri.
1. If animal is prematurely terminated after CMR business hours, 8 am to 4:30 pm
Mon-Fri, then CMR should be notified and carcass should be stored at designated freezer facility.
2. Research lab staff member must bring animal carcass with corresponding paperwork to ORSS staff (or following business day if animal is terminated prematurely). ORSS shall provide blank templates that should be recorded prior to disposal. Carcasses must follow the following precautions:
no radioactive labels inside or outside of bag
be double-bagged
should have provided ID tag attached to bag
should be disposed of properly while ORSS is present.
3. ORSS will survey animal with you while CMR staff is present and place animal in corresponding storage freezer. Animals will be placed in designated plastic tubs.
4. Animal carcass will remain in presence of ORSS monitoring until 10 half-lives have passed.
5. ORSS will transfer all decayed carcasses (after 10 half- lives) back to CMR for proper disposal as non-radioactive waste. CMR will receive written verification by
ORSS as to final survey as non- radioactive & disposal of carcass.
2
B. Bedding disposal
Radioactive microspheres: if animal is injected, there is no need to process waste. As a precaution, CMR should survey animal bedding with Geiger-Muller (GM) survey meter prior to disposal as a precaution for the possibility of microspheres being excreted by animal prior to being euthanized.
Small rodents administered with radioisotopes: Researchers should contact ORSS prior to termination for proper disposal procedure, ORSS will determine whether there is exemption. ORSS if deemed radioactive shall keep small rodent bedding within MSB
A665 radioactive waste room till 10 half-lives is completed. Bedding must also follow same precautions as above.
C. Cage sanitation
Prior to sanitization of cages for animals administered with radioisotopes,
Researchers/CMR staff must survey with GM survey meter. If radioactivity is found within cages, please contact ORSS for decontamination procedures. ORSS also has a small cage washer within MSB A665 for such circumstances.
D. Personal Protection Equipment disposal
All radioactive workers (Researchers or CMR staff) while working with animals administered isotopes other than (C14, H3, & S35) are required to attend radiation safety training & maintain a radiation dosimeter. Please contact ORSS administrator for training schedules.
3