pola27101-sup-0001-suppinfo
advertisement

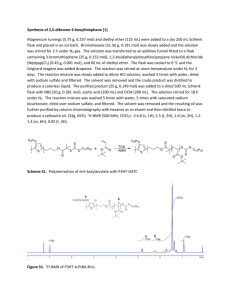
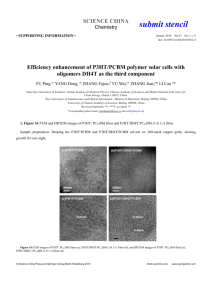
Electronic Supplementary Information For 5 Positioning Lithium Ions by Host–Guest Chemistry Combined with SelfAssembly Using a Thiophene-Based All-Conjugated Amphiphilic Block Copolymer 10 Min Jeong Im,‡ Byung Joon Moon,‡ Gang-Young Lee, Sung Yun Son and Taiho Park* Pohang University of Science and Technology, San 31, Nam-gu, Pohang, Kyoungbuk, Korea. Fax: 82-54-279-8298; Tel: 82-54-279-2394; E-mail: taihopark@postech.ac.kr 15 * Corresponding author: taihopark@postech.ac.kr ‡ These authors are equally contributed to this work. Contents 20 Experimental details and measurements. Scheme 1. Synthetic routes for well-defined structure of P3HT and BP93 with narrow molecular weight distributions. Figure S1. UV-Vis absorption spectra of P3HT and PC61BM with and without lithium salt in CHCl3 and CH3CN (99:1 v/v). 25 Figure S2. UV-Vis absorption spectra of P3HT with and without lithium salt in film. Figure S3. UV-Vis absorption spectra of a blend of P3HT and PC61BM with and without lithium salt in film. Figure S4. Height AFM images of polymer blends: P3HT+BP93:PC61BM with various amounts of lithium salts. Image size: 1ⅹ1 m. Thermal treatment at 150°C for 15 minutes. Polymer: PC61BM = 30 1:1 w/w. Figure S5. Contact angles of P3HT, BP93, PC61BM, a mixture of P3HT and BP93 (5 wt%) and blends of P3HT and PC61BM with and without BP93 (5 wt%). Figure S6. Comparison of J-V characteristics of organic solar cells: P3HT+BP93(5 wt%):PC61BM with (-●-) and without lithium salt (-▼-). 35 Experimental section: Materials. [6,6]-Phenyl –C61 butyric acid methyl ester (PC61BM) was purchased from Nano-C. All 5 other starting materials were purchased from Aldrich Chemical Co. and were used without further purification. PEDOT:PSS (poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate)) was bought from Bayer AG (Grade: PEDOT P VP AI 4083). Poly(3-hexylthiophene) (P3HT) and BP93 were synthesized employing a Grignard metathesis (GRIM) polymerization (Scheme S1) and characterization data were reported in our previous paper.3 2-Bromo-3-substituent-5-iodothiophene (1 10 and 4) was used to obtain a well-defined structure of P3HT instead of 2,5-dibromo-3-substituent. Gel permeation chromatgraphy showed narrow molecular weight distributions of 29 kDa (Mw/Mn = 1.07) for P3HT and 17.7 kDa (Mw/Mn = 1.06) for BP93 against polystyrene standards using CHCl3 as the eluent. 15 Scheme 1. Synthetic routes for well-defined structure of P3HT and BP93 with narrow molecular weight distributions. Characterization. 1H spectra were recorded on a Bruker DPX-300 (300 MHz) FT NMR system 20 operating at 300 MHz. The patterns of x-ray diffraction were measured by Rigaku X-ray diffractometer (D/MAX-2500). The target material of x-ray is copper (Cu) with the wavelength of 1.54 Å. The x-ray power is 40 kV/ 100 mA. The scanning mode is 2 theta of film mode. The scan rate is 4 deg/min. The surface morphology was scanned by Atomic Force Microscope of VEECO Dimension 3100 equipped with Nanoscope V (ver. 7.0). Number-average (Mn) and weight-average (Mw) molecular weights were determined by a size exclusion chromatography (SEC) with SHIMADZU LC solution with chloroform eluent using a calibration curve of polystyrene standards. The UV-Vis absorption spectra were measured with a Mecasys Optizen Pop UV/Vis spectrophotometer and photoluminescence (PL) spectra of the copolymers were measured on a Jasco FP-6500 spectrometer. 5 The surface morphology was scanned by Atomic Force Microscope of VEECO Dimension 3100 equipped with Nanoscope V (ver. 7.0). Measurements of contact angle and calculation of surface energy. Contact angles were measured using goniometry method (DSA-10T, KRUSS GmbH). The surface energy (γs) can be calculated from 10 contact angles as Owens, Wendt geometric mean using two different known probe liquids (deionized water and glycerol) Measurements of Mobilities. Hole-only devices of the polymers were fabricated on the prepatterned ITO substrates. After cleaning the ITO substrates with 2-propanol, acetone, ethanol and 215 propanol, UV-ozone treatment was applied for 10 minutes. PEDOT:PSS (PEDOTP VP AI 4083, Bayer AG) was spin-coated from an aqueous dispersion phase to a layer of 30 nm thick. The coated substrates were then baked at 150 ℃ for 10 minutes. After baking, a solution of the polymer in dichlorobenzene was spin-cast above the PEDOT:PSS layer to a thickness of ~70 nm, and the samples were dried for 30 minutes in the closed petri-dish. The Au electrode was deposited by thermal 20 evaporation under a 10-6 Torr of the vacuum condition. The space charge-limited current (SCLC) method was employed to get the hole mobility of the materials. The SCLS mobilities were driven by the Mott-Gurney square law. J = 90rmhV 2/8L3 25 Where J is the current density, L is the film thickness of the active layer, mh is the hole mobility, er is the relative dielectric constant of the transport medium, e0 is the permittivity of free space (8.85 10-12 Fm-1), V is the internal voltage in the device and V=Vappl- Vbi, where Vappl is the applied voltage to the 30 device, Vr is the voltage drop due to contact resistance and series resistance across the electrodes, and Vbi is the built-in voltage originated from the relative work function difference of the two electrodes. The Vbi can be determined from the transition between the ohmic region and the SCLC region. Fabrication of Photovoltaic Devices. ITO/PEDOT:PSS/Polymer:PC61BM/Ca/Al The organic photovoltaic cells with PEDOT:PSS (poly(3,4-ethylene architecture, used an dioxythiophene):poly(styrene sulfonate))-coated indium tin oxide (ITO) glass substrates as transparent 5 anode and aluminum as the cathode. Commercially available patterned ITO was washed with the sequence of 2-propanol, acetone, and ethanol. The ITO substrates were blown using N2 gases for drying. After treatment with UV-ozone for improving hydrophilicity with the following layer, a 30 nm layer of PEDOT:PSS was spin-coated. Then the devices were transferred to N2 filled glove box for active layer deposition. The active solution was dissolved in o-dichlorobenzene over 10 hours before 10 the fabrication. The ratio of the active solution (the blends of polymer and PC 61BM) is 1:1 w/w. The concentration of BP93 to blend solution was 5 wt %. The blending of P3HT:PC61BM with BP93 was also conducted over 10 hours in N2 filled glove box. Spin-coating for the active layer was targeted toward the thickness of ~100 nm. The top electrode (Ca/Al, 3 nm/100 nm) was deposited via thermal evaporation through a shadow mask under the pressure of 7.0 10-7 Torr. 15 Photovoltaic Measurements. Photovoltaic measurements of the OPVs were carried out using a Keithley 2400 digital source meter controlled by a computer under illumination of simulated AM 1.5 G solar light from an AM 1.5 solar simulator (Newport, M-91190A, with a 450 W xenon lamp). Power was regulated to the AM 1.5 G solar standard by using a reference Si photodiode equipped with a 20 color-matched filter (KG-3, Schott) in order to reduce the mismatch in the region of 350-750 nm between the simulated light and AM 1.5 G to less than 4 %. Figure S1. UV-Vis absorption spectra of P3HT and PC61BM with and without lithium salt in CHCl3 and CH3CN (99:1 v/v). 5 Figure S2. UV-Vis absorption spectra of P3HT with and without lithium salt in film. Figure S3. UV-Vis absorption spectra of a blend of P3HT and PC61BM with and without lithium salt in film. 5 Figure S4. Height AFM images of polymer blends: P3HT+BP93:PC61BM with various amounts of lithium salts. Image size: 1ⅹ1 m. Thermal treatment at 150°C for 15 minutes. Polymer: PC61BM = 1:1 w/w. Figure S5. Contact angles of P3HT, BP93, PC61BM, a mixture of P3HT and BP93 (5 wt%) and blends of P3HT and PC61BM with and without BP93 (5 wt%). 5 Figure S6. Comparison of J-V characteristics of organic solar cells: P3HT+BP93(5 wt%):PC61BM with (-●-) and without lithium salt (-▼-). 10