H1`-H2`
advertisement
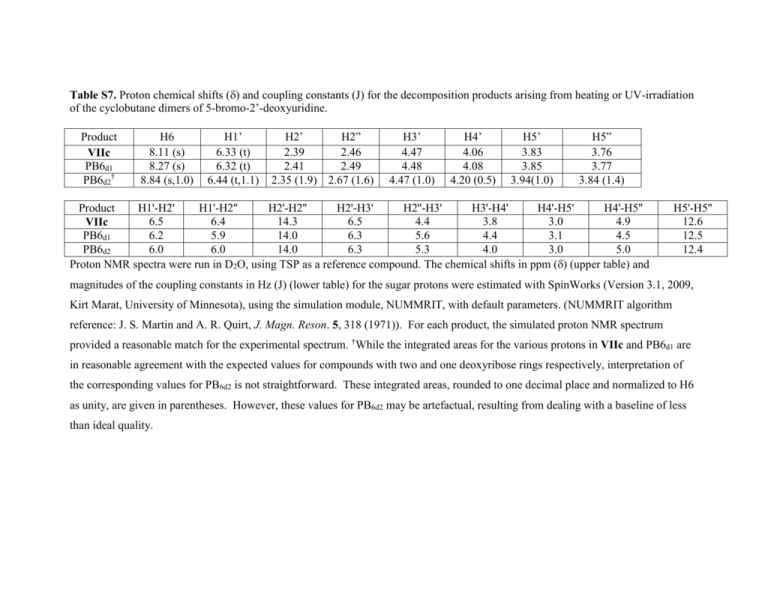
Table S7. Proton chemical shifts () and coupling constants (J) for the decomposition products arising from heating or UV-irradiation of the cyclobutane dimers of 5-bromo-2’-deoxyuridine. Product VIIc PB6d1 PB6d2† H6 8.11 (s) 8.27 (s) 8.84 (s,1.0) H1’ 6.33 (t) 6.32 (t) 6.44 (t,1.1) H2’ H2” 2.39 2.46 2.41 2.49 2.35 (1.9) 2.67 (1.6) H3’ 4.47 4.48 4.47 (1.0) H4’ 4.06 4.08 4.20 (0.5) H5’ 3.83 3.85 3.94(1.0) H5” 3.76 3.77 3.84 (1.4) Product H1'-H2' H1'-H2'' H2'-H2'' H2'-H3' H2''-H3' H3'-H4' H4'-H5' H4'-H5'' 6.5 6.4 14.3 6.5 4.4 3.8 3.0 4.9 VIIc PB6d1 6.2 5.9 14.0 6.3 5.6 4.4 3.1 4.5 PB6d2 6.0 6.0 14.0 6.3 5.3 4.0 3.0 5.0 Proton NMR spectra were run in D2O, using TSP as a reference compound. The chemical shifts in ppm () (upper table) and H5'-H5'' 12.6 12.5 12.4 magnitudes of the coupling constants in Hz (J) (lower table) for the sugar protons were estimated with SpinWorks (Version 3.1, 2009, Kirt Marat, University of Minnesota), using the simulation module, NUMMRIT, with default parameters. (NUMMRIT algorithm reference: J. S. Martin and A. R. Quirt, J. Magn. Reson. 5, 318 (1971)). For each product, the simulated proton NMR spectrum provided a reasonable match for the experimental spectrum. †While the integrated areas for the various protons in VIIc and PB6d1 are in reasonable agreement with the expected values for compounds with two and one deoxyribose rings respectively, interpretation of the corresponding values for PB6d2 is not straightforward. These integrated areas, rounded to one decimal place and normalized to H6 as unity, are given in parentheses. However, these values for PB6d2 may be artefactual, resulting from dealing with a baseline of less than ideal quality.











