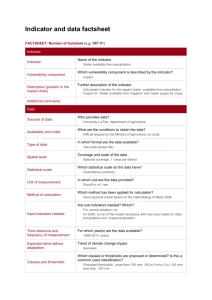
WHO User Manual: Laboratory Assessment Tool
advertisement

User Manual of the Laboratory Assessment Tool WHO/CSR/Lyon 23/06/04 WHO OMS Table of contents Table of contents ........................................................................... 1 Table of figures.............................................................................. 2 Acronyms ..................................................................................... 2 1- Introduction .............................................................................. 3 2- Presentation of the tool................................................................. 3 3- The assessment process................................................................ 4 4- National consensus on laboratory equipments ..................................... 7 5- General guidelines in completing each module ..................................... 9 6- Module 1: General facts ............................................................... 10 7- Module 2: Biosafety .................................................................... 12 8- Module 3: Sampling .................................................................... 15 9- Module 4: Equipment .................................................................. 18 10- Module 5: Reagents .................................................................. 19 11- Module 6: tests ........................................................................ 22 12- Module 7: Laboratory staff & working time ....................................... 25 13- Module 8: Total quality ............................................................... 27 14- Module 9: Reporting, analysis and communication .............................. 30 15- Module 10: outbreaks ................................................................ 31 16- Analysis of the results and summary .............................................. 33 17- Un-protecting & Editing LAT......................................................... 37 Appendix 1: indicators calculation ....................................................... 39 User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 1/52 Table of figures Figure 1 : the various worksheets............................................................................. 3 Figure 2 : equipment list based on the level of the laboratory ........................................... 8 Figure 3: revealing additional information hidden in the cells ............................................ 9 Figure 4: limited possibility for answers (here Y, N and na) ............................................ 10 Figure 5 : error message in case of unauthorized value ................................................ 10 Figure 6 : upper part of the module about general facts................................................ 11 Figure 7 : part of sterilization logbook, Plovdiv, Bulgaria, 2003 ........................................ 14 Figure 8 : WHO biosafety guideline ......................................................................... 15 Figure 9 : filing in of the equipment module .............................................................. 19 Figure 10 : screening test for diphtheria not needed .................................................... 23 Figure 11 : screening test for diphtheria not done ....................................................... 23 Figure 12 : screening test for diphtheria done ............................................................ 23 Figure 13 : confirmation test for dysenteria ............................................................... 24 Figure 14 : confirmation test for tuberculosis not done ................................................. 25 Figure 15 : critical thinking toward samples during night and week end ............................. 27 Figure 16 : sheet containing all indicators and their graphic representation ......................... 34 Figure 17: general indicator calculation, including the 10 modules ................................... 34 Figure 18: calculation of the general indicator without the modules 2 and 8 ........................ 35 Figure 19: weighted indicator sheet ........................................................................ 35 Figure 20: list of available coefficients ..................................................................... 36 Figure 21: general un-weighed indicator .................................................................. 36 Figure 22: difference in general un-weighed indicator and general weighed indicator. ............ 36 Figure 23 : active sheet unprotected ....................................................................... 37 Figure 24 : right board un-removed ........................................................................ 38 Figure 25 : right board removed once unprotected, calculations can be seen ...................... 38 Acronyms AST: Antibiotic Susceptibility Testing CSR: Communicable disease Surveillance and Response CCL: Core Capacities of Laboratories EQC: External Quality Control IDSR: Integrated Disease Surveillance and Response IHR: International Health Regulation IQC: Internal Quality Control LAT: Laboratory Assessment Tool OEL: Outils d’Evaluation des Laboratoires PHL: Public Health Laboratory SOP: Standardized Operating Procedure CSR/LYO/LAB unit: Medical staff: Unit coordinator: Dr Bradford Kay Training responsible: Dr Philippe Dubois Quality assurance responsible: Dr Antoine Pierson Country activity responsible: Dr Mohamed Youssef Country follow-up responsible: Dr Sébastien Cognat Lab management responsible: Dr Isabelle Bergeri Distance learning and tool conception staff: Ms Anouk Berger Administrative staff: Ms Isabelle Battaglia Ms Anjelika Efimova Note: Mr. Hojoon Sohn did take active part in reviewing and editing this tool User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 2/52 1- Introduction The Laboratory Assessment Tool or « LAT », also called « outil d’évaluation des laboratoires (OEL) » in French, is an evaluation tool designed to: Assess a microbiological lab in a standardized way Automatically generate numerical indicators related to laboratory capacities In different parts called « modules » Easily follow the improvement of the same laboratory over time Perform an evaluation based on the technical and administrative level of the laboratory (reference, intermediate, periphery) The current edition of the assessment is an MS Excel file, and is ran only using calculations (no macros). Absence of macros enables the LAT to be used on any computer, independent of the operating system language. This assessment tool has been developed and revised by the WHO/CSR/Lyon unit in 2003 and 2004 with the comments and input of many contributors. This current version has been field tested in Bulgaria, Iran, India, and in Burkina Faso. The principal designers and editors of this assessment tool are Dr. Antoine Pierson and Dr. Augusto Pinto. 2- Presentation of the tool The file includes sixteen worksheets, titled in English. Altering or renaming of these titles may result in calculation error and possibly compromise the interpretation of the data. Titles and descriptions of each worksheet Figure 1 : the various worksheets Indicator summary: A full summary of evaluation and assessment of the laboratory with LAT. All indicators: A visual summary of the evaluation of each indicators. 3 pages when printed. Specific Modules 1-general: includes questions concerning the details of the laboratory facility being assessed and the respondent. This section evaluates User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 3/52 laboratory building conditions, water and electricity supply and general activities of the laboratory. 2-biosafety: includes questions about biosafety practices, waste management and security of the lab being assessed 3-sampling: includes questions regarding the laboratory’s sampling procedures, request forms, and specimen recording procedures. 4-equipment: includes questions targeting the equipment status of the laboratory. Recommended equipments (both quantity and types) are listed in the « Nat. Consensus on equipment » module. 5-reagents: gathers information concerning reagents and supplies utilized in the laboratory, their management, and preparation. This module also includes questions concerning financial resources for purchasing reagents and supplies. 6-tests: This large module assesses the laboratory’s diagnostic test and procedures. 7-staff: This module concerns staff and their working conditions at the laboratory. (E.g. the number of days and hours of opening, procedures in emergency cases handled outside the opening hours). 8-quality: This module is dedicated to the examination of the overall quality procedures of the laboratory: quality assurance, internal and external quality control, analysis procedures, preventive maintenance and repair of laboratory equipment. 9-reports: Communications capabilities of the laboratory are assessed in this module, which includes reporting of priority diseases to the public health authorities, data archives, monthly reports and data backup procedures and practices. 10-outbreak: This module examines the laboratory’s activities in disease outbreak investigations. Nat. Consensus on equipment: This module is a summary of both essential and optimum level of equipments (both number and type) recommended in the laboratory classified by the level of the laboratory. This sheet is used as a reference for interpretation of the results from module 4 (equipment module). weighed summary: A full summary of the assessment on which you can weigh (3 levels) any of the ten modules for the summary indicator calculation weighed indicators: A visual summary of the evaluation of each indicators, that can be weighed (3 levels) to calculate the module summary 3- The assessment process IMPORTANT NOTE: It is imperative that the assessment is carried out by a microbiologist or a person who has in-depth knowledge in laboratory assessment User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 4/52 (includes those who have prior experience in laboratory assessment) and understands the general laboratory operations. 1) Recommended length of the assessment A complete assessment of a laboratory should take at least half a day for a reference laboratory and should take about 2~3 hours for a peripheral laboratory. This assessment must be carried out during opening hours, in order to be able to observe staff at work. Aside from bringing both electronic and paper version of LAT, it is necessary to have a notebook and a camera (ideally digital) for documentation purpose. 2) Approach to the assessment and securing cooperation from the laboratory Before you begin the assessment of the laboratory, it is recommended to the assessor explain the main purpose of the assessment. You MUST point out that this is not a control process that may lead to punitive measures. It should also be mentioned that there is an agreement with the national authorities that the assessment will indeed NOT be used for punitive measures. The followings are recommended to be highlighted at the conclusion of the assessment: Strengths of the laboratory, in order to highlight positive aspect of the laboratory Weaknesses of the laboratory in order to highlight future improvements of the laboratory IMPORTANT: Furthermore, it is very important to note that assessment results should follow confidentiality rules, and in establishing such a confidence climate, a quality assessment will be possible to be carried out 3) Assessment procedures and guidelines The assessor should first visit the laboratory, following the sample path: Sample or patient reception Sampling rooms Recording Microscopy Technical rooms (culture, Identification, Antibiotic Susceptibility Testing, serology, molecular biology …) Support rooms (washing room, sterilization, restroom, guard room, stock room, repair room, secretary room, offices) Look at the global cleanliness, the global organization, biosafety level while manipulating User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 5/52 In each room (if applicable), the assessor may be required to perform following tasks: Check the condition and organization of refrigerators Check the condition and organization of incubators Check the condition and organization of freezers How is the eventual Visually evaluate the condition and cleanliness of the laboratory benches and facilities Examine the condition and organization of stock shelves, cards, and expiration dates of the chemicals on the shelves Check the laboratory logbooks, patient records or information (check if they correspond to the right patient), and management of the antibiogramme results Check the eventual archives Check the condition of the microscopes (always assess one or two slides in order to check 1) Are the refrigerators clean? 2) Randomly take 3 or 4 old-looking products and check expiration dates 3) Is there an internal thermometer available? If not, are there any temperature charts available? 4) etc. 1) Look at the thermostat adjustments and eventual hemoculture bottles 2) Check the organization of Petri dishes Are there any old Petri dishes? Do all of Petri dishes have identifications? 3) Maintenance of special environment (CO2, anaerobia, microaerophila…) 4) Check how the temperature of the incubator is recorded (ideally, it is recommended to have a thermometer inside a water can in the incubator) 5) Check the overall cleanliness of the incubator serotheque organized? 1) the quality of the films and stains, 2) the condition of the microscope, and 3) the Kohler adjustment (centering of optical axes). Observe and evaluate how laboratory technicians work Check and evaluate how the laboratory waste is managed inside technical rooms. This is important information as it will tell us about the laboratory’s activities within the past 24 hours of the assessment. 1) Do they use lab coats, gloves, glasses, masks? 2) Is the bench clean? Do they disinfect the bench at the end of the work? Is regular disinfection of the lab bench likely? 1) How full are the waste containers? 2) Are the wastes regularly emptied? User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 6/52 3) Are there separate waste (lid-covered?) containers for non contaminated and contaminated wastes? 4) Is there any special solvent container (any for acids?) Etc. Advice and recommendations should be made following the observation and examination. It is important to note that these assessments are only made to improve the condition of the laboratory and understand that keeping good relations between the assessor and the laboratory personnel is vital. Hence, all recommendations and advice should be made in friendly manner. Furthermore, some comments that may be quite embarrassing or upsetting for the laboratory should only be mentioned to the respondent of the laboratory (or the person in charge). During the visit, document the facility by taking pictures of the rooms, working staff, equipments, packaging delivery procedures, etc. These pictures can aid you to illustrate and explain the conditions of the laboratory for the final report. A detail visit, normally lasting around 90 minutes for a reference laboratory, is a must as it will allow the assessor to save time and be more efficient during the utilization of the tool. It also gives the assessor an opportunity to demonstrate several key improvements to be made for the laboratory and their staff on site. Once the initial visual evaluation of the laboratory facility is completed, immediate assessment of the laboratory, using LAT is recommended. The assessor can initially use the paper version of the tool for his/her convenience, but it is recommended him/her to directly utilize and fill-in the electronic version. Utilization of the electronic version of this tool (LAT) enables immediate discussions with the respondent from the laboratory following the questions and make recommendations for future improvements of the laboratory, suggested from the indicators of LAT. 4- National consensus on laboratory equipments LAT worksheet 13 is a list of recommended equipments and their quantity based on the level of the laboratory. The current list has been carefully prepared after extensive discussions between WHO collaborators and the developer of this tool and is based on our previous laboratory assessment experiences, mostly in Africa, Middle-East and Eastern Europe. The list of equipment by level can be kept as provided in LAT However, it is highly recommended to health authorities to refine this list when assessing a certain number of laboratories in their specific country. This can provide a unique opportunity for health authorities to organize a workshop on User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 7/52 equipment that should be available for each level of public laboratories and then issue National recommendations about this important topic1. Based on the assessment information on equipments and the National consensus on laboratory equipments, LAT can automatically generate a list of recommended equipment and its quantities required or needed as well as financial parameters in purchasing these equipments for the assessed laboratory. Thus, it is IMPORTANT for the assessor to review this table before the assessment as the calculation of the indicator for the equipment module is linked to this table and depends on the level of the laboratory being assessed2. This table is listed with both ‘minimum’ and ‘optimum’ equipment quantities for each laboratory level. ‘Minimum’ is defined by the number of equipments absolutely necessary for laboratory operations and ‘Optimum’ concerns the quantity of equipments for ideal laboratory operations (figure 2). For some ‘minimum’ and ‘optimum,’ “0” signifies the following: 1) the equipment is not needed for the purpose of the laboratory (ex. disease specific or specialized laboratories) 2) the equipment is not needed for the particular laboratory level (ex. culture and serology analysis)3 Figure 2 : equipment list based on the level of the laboratory In addition to this list of equipment by level, it is also very important to get the list of staff required and the list of analysis that should be performed at each level of the laboratories 2 « 1 » is the peripheral level (1~2 laboratory staffs), « 2 » is the intermediate level (5~10), and « 3 » is the reference laboratory (11~40). 1 3 Analysis Microscopy Serology (ex. etc.) Culture ELISA, Level 1 (district) Required If needed, but not required (only for HIV analysis or other relevant disease analysis) Not recommended (outside any specific and/or special programs) Level 2 (interm.) Required Recommended (depending on the operational capabilities) Recommended User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 Level 3 (ref.) Required Required Required 8/52 5- General guidelines in completing each module To help you with assessment process and completion of LAT, additional information will be available for some selected questions and indicators in LAT. This information is hidden in the cells, but can be viewed by dragging the cursor to the cells with corners highlighted with red (Figure 2). Figure 3: revealing additional information hidden in the cells Besides the consensus about equipment, all gray cells of the modules 1 to 10 must be filled in. For each question, and for the entire tool, you have a limited number of possible answers: « Y » for yes « N » for no « na » for « non applicable », should be entered if the question isn’t applicable to the laboratory. For example, « do you diagnose tuberculosis using bacilloscopy »; if the laboratory doesn’t perform this analysis (availability of another TB specialized centre), you reply « na » and the question goes out of the indicator calculation. « 1, 2, 3, 4 or 5 » for open question with different possible answers Different values other than those above can entered when asking: o Quantity of equipment o Number of tests performed annually o Percentage of equipment coming from outside o Number of samples handled daily o etc. User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 9/52 Figure 4: limited possibility for answers (here Y, N and na) Clicking the small arrow at the right side of the cell opens a listbox with authorized values. Error message will appear as below, when trying to enter values other than the ones listed in the listbox: Figure 5 : error message in case of unauthorized value Hence, YOU CAN ONLY ENTER AN AUTHORIZED VALUE IN THE LISTBOX. At the end of each question, next to the gray cell, you can find a code similar to epi-info 6 or epi-data codes: <Y> signifies a yes/non answer, sometimes « na » # signifies that a one digit number must be entered (1, 2, 3…) ## signifies that a one or two digits number must be entered ### signifies that a one, two or three digit number must be entered IMPORTANT NOTE: If you think the answer to the question applies to both Y and N, you must SYSTEMATICALLY choose the most severe answer of the two. Examples: 1) If the answer is « yes » in few cases but « no », for most cases, you have to choose « no ». 2) For question « did somebody from the staff attend a training on biosafety before working? », if a new staff member at the laboratory attended a refresher course on biosafety, and not a full training course with handouts, you must choose « no » for the question This systematical « severity » will allow a calibration between the different assessors and may allow their respective assessments to be more comparable. 6- Module 1: General facts The upper part of this module has to be filled in carefully as these will be the header of the printable reports. User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 10/52 Figure 6 : upper part of the module about general facts Please pay careful attention when entering the level of the laboratory being assessed as this number is used in the calculation of several indicators in LAT (circled in figure 5). Note: « 1 » is the peripheral level, « 2 » is the intermediate level, and « 3 » is the reference laboratory Building conditions There are three questions in this section concerning the building conditions including questions regarding roof/ceiling, wall, and the ground of the laboratory: o Answer « 3 » if the state of the building is very good (new or close to new or well maintained) o Answer « 2 » if problems exist (water infiltration, walls spoiled or attacked by termites) but won’t cause major problems o Answer « 1 » if major infiltrations are present: walls showing major structural defect or breakdown, the ground is damaged, compromising the working quality inside the laboratory Fluids supply Enter the % of water and electricity availability, practically: o 100% if you don’t have any shortage o 90% if shortages are rare (1 to 2 time monthly) o 70% if shortages are regular (once a week) o 50% if shortages are systematic (more than once a week) o 30% if shortages are occurring daily For the generator, answer « Y » if the electric generator is present and functions properly, AND if its supply in gasoline is done regularly. Otherwise, answer « N » For the safe sewage disposal method, verify whether or not the laboratory implements a system for separating or disinfecting hazardous and non hazardous liquid waste before draining out to the common sewage Laboratory rooms User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 11/52 1) Enter the total number of rooms inside the laboratory, including technical rooms, reception, sampling, offices, stock, rest room, guard room. Do not count toilets. 2) Enter after the number of benched rooms, and the number of used benched rooms. For example, enter 3, if the laboratory has a total of 10 benched rooms, but with only 3 functional or being used. NOTE: If an office is equipped with laboratory benches that are not used for laboratory purposes, you have to count the room as “non used benched rooms” and exclude them from the total number of “number of rooms in current usage.” Communication possibilities Answer « Y » or « N » for each question concerning the communication means in the laboratory facility. NOTE: In order to answer « Y » for certain questions, the laboratory must meet the following: Telephone and fax are present inside the laboratory The computer(s) (At least MS Windows 98 operated or Apple OS 9.01 version is good enough) is (are) present inside the laboratory Internet connection/access is available throughout the facility for regular access for the laboratory personnel (connections can be localized at another location). Functional national postal services are available at the facility. Coverage of infectious diseases Fill « Y » or « N » for each question. 1) The part « virology » concerns the « real » virology with cell culture and virological diagnosis. You should select answers for serology separately. 2) Answer « na » for tuberculosis, parasitology and mycology, ONLY if these specialties are covered by another reference centre, otherwise, answer « Y » or « N ». For molecular biology analysis, answer « na » for level 1 and 2 laboratories. For reference laboratory, answer « Y » if the unit is functional, and « N » if the unit is non functional or not present. NOTE: Reference laboratories are recommended to have a molecular biology analysis unit. 7- Module 2: Biosafety User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 12/52 Use of protective gears For these questions, your answers should be based on your observations of the laboratory and their staff. For each protective gear (labcoat, glove, mask and glasses), you should respond « 1 » if the laboratory staff never uses them, « 2 » if rarely uses them, and « 3 » if always uses them. During the visit, pay close attention to the use of the gloves. Availability of safety procedures Questions in this section concern WRITTEN or PRINTED procedures regarding biosafety in the laboratory. Ask the staff member to show you these procedures. Visual verification of these procedures is recommended. Training in biosafety Questions in this section concern the training procedures in biosafety at the laboratory being assessed. Each question addresses safety training in following recommended categories: o Security while manipulating laboratory samples o Classification of different classes of micro-organisms o Security while sampling o Methods in Packaging and triple-packaging of a sample o Using disinfectants and procedures in disinfections o Waste management Note: Each training session should be done with proper handouts, containing necessary information about each training subject. The biosafety training sessions can be carried out by either in-lab professionals or professionals from outside the laboratory Safety conditions For answering any biosafety conditions of the laboratory, following observations should be made: o Availability of hypo chlorite or similar liquid disinfectants at the laboratory benches and at the washing room4. * Cresols (liquid or solid) or Lysol (50% cresol in aqueous solution). * Hypochlorite (household bleach) * Calcium hypochlorite * Chloramine-T * Calcium hydroxide solution (not suitable for disinfecting stools from patients with TB) * Quaternary ammonium compounds (QUATS) * Alcohols (ethanol, isopropanol) or other alcohol based disinfectants 4 User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 13/52 o Availability of separate sink for washing hands (please check if there are any biological staining traces!). o Check the condition of the laboratory benches and its materials (wood or plastic benches?). Note: Benches made of resin, glass, ceramic or stone are recommended as they are resistant of chemicals and other biological solvents and are easily washable. Disinfection/sterilization of laboratory equipments 1) Centrifuges It is imperative that disinfection of the centrifuge is done when the centrifuge is contaminated with samples from a tube break or other cause. It is recommended that the centrifuges are cleaned and disinfected regularly, at least once a month. If these two conditions are met, answer « Y », otherwise, answer « N ». 2) Incubators Likewise, disinfection of the incubators in the laboratory must be done when the incubator is contaminated (or contamination is suspected). It is also recommended that incubators are disinfected and cleaned every 60 days regardless of contamination. In LAT, answer « Y » if these two criteria are met. Otherwise, answer « N ». 3) Sterilization indicators Sterilization indicators should be used for EACH sterilization. You should ask the laboratory respondent to see the strips being used at the laboratory for inspection. If the laboratory does not use such strips, you should recommend the use of a sterilization strips and a sterilization logbook for to document each sterilization (ex. Plateau temperature). 3 2 4 5 6 1 Figure 7 : part of sterilization logbook, Plovdiv, Bulgaria, 2003 The logbook should contain the following categories: 1. Date and list of the sterilized items User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 14/52 2. 3. 4. 5. 6. Time of beginning and ending of sterilization Plateau temperature Number of baskets (being sterilized) Strip glued, verifying sterilization. Signature of the officer Waste disposal Check the following for waste disposals in the facility: 1) Availability of two separate waste containers for infectious and noninfectious wastes. For infectious waste container, please check if the waste container has a lid. 2) Availability of broken glass/sharp object waste containers in the sampling rooms. 3) Check if there are any unsecured ‘waste’ bowls or small static bench cans (static meaning not emptied regularly) in the laboratory. Also check if the waste containers are made in materials other than rigid plastic (wood, iron, etc.). NOTE: Some African laboratories may use large jerrican as solvent containers. Documents in biosafety Relevant and updated biosafety guide must be present in order to be able to check « Y » to this question. For the WHO security manual, the lab must possess one of the following three manuals (shown in the picture below – please show the picture of these books to the respondent). Figure 8 : WHO biosafety guideline 8- Module 3: Sampling User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 15/52 Laboratory capacity in sample processing AND Quality of samples received Answering questions in these two sections enable the LAT to assess the main problems concerning sample received by the laboratory: o Wrong package means that a regular safe package (triple package for example) wasn't use for the transport. This can also mean that some leakage happens regularly or any other package failure o Wrong conservation means that samples are not delivered at the right temperature (frozen, cold or at 37°C) o Wrong transport media: for example CSF in Cary Blair transport media, or no transport media when needed o Wrong identification: samples for HIV labeled as TB, etc., incorrect or wrong identification of the patient… o Delay in sample delivery: samples delivered with a delay prohibiting a correct further analysis Availability of sampling procedures o First four questions in this section concerns written sampling procedures used by the laboratory. The respondent from the laboratory must be able to show you these documents in order to check « Y » to these four questions. o Next question only refers to the countries that have more than one official language (ex. India, Cameroon, Namibia, China, etc.). In these countries, it is common to have procedures written in a language that may be different from the language used by the technicians or laboratory personnel. If this is the case, answer « N ». For countries with one official language, such as francophone countries in Africa (where medical training and teaching is done in French), answer « na ». Staff Access to the sampling procedures: Staff access to these procedures is critical and should be permanently accessible. Answer « N » if these procedures are kept in a closed office or not available. Review of Sampling Procedures by Clinician: Sometimes lab samples are collected by non-laboratory personnel or clinician. Hence, if the laboratory has standardized written or printed procedures for sampling, availability of these sampling procedures to the clinician can significantly improve the quality of the samples. This is the same in case of biologists/physicians collecting the sample. If the sampling procedures are NOT available to these people, answer « N ». Quality of the request form User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 16/52 The best way to answer these questions is to have a sample request form in hand. Please ask the respondent a copy of the sample request form being used in the laboratory. Specifically look whether or not the form has sampling time and date section. NOTE: If the form is meningitis and/or hemoculture specific, temperature column must be present. If this is not the case, answer « N » for the temperature question. Answer « na » if the form is not specific for meningitis or hemoculture specimen. 5 Critical thinking about the samples This question is to assess laboratory’s logistical procedure in handling samples with limited information. LAT has three possible responses labeled 1 to 3. Expected answer from the respondent is the 3 rd one (‘TRY TO CONSULT THE SENDER OR THE PATIENT’). When asking the question, give the respondent an opportunity to answer independently (without knowing the response choices). If the answer significantly differs from one of three answer choices or the respondent has trouble answering, then, ask the respondent to choose from the three possible responses. Identification of Specimens To answer questions regarding identification of specimens, you should first refer to the logbook of the laboratory being assessed. Answer « Y », « N », or « na » for each question based on your examination of the logbook. Macroscopic examination For the first question, ask the staff member or respondent if macroscopic examination is done on all samples received and analyzed5. If so, verify this in the logbook and answer « Y » to both questions. If macroscopic examination is not recorded in the logbook, answer « N » for the second question. Sample destruction This question is to assess laboratory’s sample management after testing (ex. to verify if the results from the samples are obtained and verified before being discarded and to check how long the samples are kept if retesting may be necessary). When asking the question, give the respondent an opportunity to answer independently (without knowing the response choices). If the answer significantly differs from one of three answer choices or the respondent has trouble answering, then, ask the respondent to choose from the three possible responses. Stool, CSF, serum, urine, etc. User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 17/52 Specimen tracking Answering first two questions in this section in « Y » will require you to examine the laboratory logbook. The logbook should be organized so that one may be able to find records of specimen by the following: o Patient’s name (Can you find the specimen or test results by a patient’s name?) This is possible only if the laboratory keeps a separate book with alphabetical list of patient names. Answer « N » if this book is not present. o Sampling Date (Can you find the specimen or test results by sampling date?) This method is may be the best way to find samples, if the logbook is well organized. To answer this question, ask the respondent to find a sample by giving a random date. Answer accordingly based on the result the respondent’s performance. o Results of the test (Can you find the specimen record from a test result?) Search by the result of the test is possible only if the laboratory keeps a separate book containing alphabetic listing of patients with positive test results. Answer « N » if this book is not present. 9- Module 4: Equipment This module is linked to the national consensus about equipment by level (see page 7). When filling in this module, count the number of FUNCTIONING6 equipment, easily ACCESSIBLES7 for the microbiology laboratory. This module is directly linked to the national database on recommended laboratory equipments based on the level of the laboratory.8 When completing this module, only count the number of FUNCTIONING equipments in the laboratory and ACCESSIBLES in the microbiology laboratory. Automated comparison with the national database for the laboratory equipments, done by the program, will generate recommended list and amount of equipments needed to meet the National standards. Financial requirements in obtaining recommended laboratory equipment can also be calculated in this module. In order to do this, you must enter the price of EACH equipment (in USD) in the « Functioning » means the equipment is in working condition on the day of the assessment. 7 Only count hematology or parasitology equipment only if Located in the same premises The equipment can be used without constraints by the microbiology laboratory, meaning no official request is necessary to use the equipment. 8 refer to page 7 of this manual and the Nat. Consensus of equipment module in LAT 6 User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 18/52 column labeled « average price ». The result will appear at the bottom of the page. NOTE 1: All equipment prices are listed as average prices in US dollars and it takes account of the costs for shipping and handling and 10% of total equipment price for purchasing spare parts at the time of initial order (spare parts for the equipment can be best purchased when bundled with the initial order of the equipment as it (may) allow additional discounts). NOTE 2: There are two parts to this calculation: TOTAL and BASIC. Total refers to the entire equipment recommended while BASIC refers to the equipments essential for microbiology laboratory. Following are the list of equipments that are included in the BASIC group: autoclave fridge scale freezer –20° microscope incubator (large and small) Candle jar water distiller ELISA chain water bath NOTE 3: Please note the following when entering information about glassware: 0- Don’t have any laboratory glassware 1- Shortage (laboratory glassware is available, but not enough to cope with the capacity of the laboratory). 2- Minimum (the laboratory has just enough glassware for normal operation). 3- Optimal (the laboratory has more than enough glassware for its capacity). Figure 9 : filing in of the equipment module 10- Module 5: Reagents In-lab preparation of reagents This section concerns any media or reagents that are prepared in the laboratory oppose to pre-made products from companies. If the laboratory prepares (some of) its own reagents/media, then check the preparation of following three examples: User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 19/52 o « Gram »: Ask if the laboratory receives the stains ready-made (costly, but convenient and quality assured) or in powder form (quality may depend on the preparation procedure and conditions). Answer « N » if lab purchases ready-made stains. o « Blood agar »: While chocolate agar (cooked blood) is easy to prepare by mixing hemoglobin in powder, blood agar requires use of fresh blood. Hence, it requires easy access to fresh blood supply (e.g. blood from a sheep). Human blood is not recommended in preparation for blood agar. Answer « Y » if sheep blood is easily available to the lab (e.g. a large number of African laboratories own 2 or 3 sheep at or near the facility for this purpose). o BCP agar (Bromo Crésol Pourpre) or McConkey agar both are basic media and can be easily prepared at the laboratory. Answer « Y » if the laboratory prepares its own BCP agar or McConkey agar. The part about QC of the home made media has to be strictly marked: o Gram : stain has to be controlled Visually (no sediment, precipitate or silt) Using known Gram + and – strains (ATCC for example) and adjusting if relevant, the times for the staining and decolorizing steps. o Home-made media Visual tests: aspect, color and smelling Sterility tests: for EACH batch, during 48 hours, written on a media preparation logbook Growing tests: to be performed at least for selective media (TCBS, SS, HEK, chromogenic media…) If one part of these controls is lacking, answer « N » It is important to be thorough in checking the quality of the media/reagents made in the laboratory. Please check the following: o Gram Visually check if there are sediments, precipitates, or silts. Comparing with the standard Gram positive and negative strains samples (ex. ATTC or Pasteur Institute standards), examine staining and decolorizing steps. o Lab made media Visual tests: aspect, color, and smell Check if the media is sterile: For EACH batch of media (a sample of each batch incubated for 48 hours), please check if the media preparation is documented in the relevant laboratory logbook and verify the date labeled on the bottle. Perform growing test: It is recommended to perform growing for at least the following list of selective media: TCBS, SS, HEK, chromogenic media, etc. User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 20/52 Reagent management 1) It is required for each laboratory to have AT LEAST stock cards for the laboratory’s stock management. Answer « Y » to the first question if the laboratory has stock cards (you need to verify this) or a system similar to stock cards. Answer « na » for the first question if the laboratory manages the stock of its reagents electronically, for central level laboratories, it is highly recommended to have an electronical reagent stock management system.9 2) A simple UP-TO-DATE MS EXCEL worksheet version of stock management system is good enough to count as an electronic management of the reagents in the laboratory. If this applies to the laboratory (ex. MS Excel file), you can answer « Y » for the second question in this section. 3) It is highly recommended that laboratories keep some sort of logbook for expired reagents. If any expired reagents or media is found in the laboratory, answer « N ». NOTE: Some laboratories keep expired reagents for teaching purposes and keep them in a separate location with clear labels. If the laboratory does not keep a logbook for expired reagents or have a separate location for stocking expired reagents, do recommend the respondent or other laboratory personnel to keep a logbook. A monthly inventory or check-up of the expired reagent is recommended. 4) Check if the opening date is recorded on the reagent bottles or containers. Answer « N » if there is reagent containers without opening date recorded on the container as this process should be universal for all reagents used in the laboratory. 5) For this question, the laboratory should perform at least 2 major inventories of their reagents and media kept in the laboratory. If this is the case, answer « Y », otherwise, answer « N ». Funding for purchase of reagents used in the laboratory ‘Dedicated funds’ means that a specific line inside the budget of the institution, line managed by the director of the lab. If you have to make your orders through administration, this is not a dedicated line and you will have to answer « N » The 3 other questions can be interpreted as well for funds or for reagents donation, if these donations are regular. If one of these 2 conditions is met, answer « Y » Softlab® is a software specifically designed for laboratory stock management, for any details, contact Dr. Antoine Pierson (piersona@lyon.who.int), WHO/CSR/Lyon Office 9 User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 21/52 These set of questions are useful in determining the sources of fund used for purchasing reagents at the laboratory. “Dedicated funds” means a specific line of funds included in the budget of the laboratory or the institution managed by the director of the laboratory. o For ONLY THE FIRST QUESTION, answer « Y » if the funding for reagent is part of the institution’s budget. o For the rest of the questions in this section, answer « Y » if the donations or funding is permanent or regular for relevant organization. Use of expired reagents This is simple to answer. Be cautious in choosing the « 3–never » answer. If the laboratory uses ANY TYPES of expired reagents (even if it is very rarely used), DO NOT select this answer. Availability of various reagents There are 8 sections (a total of 42 questions) specifically designed to assess the availability of reagents/antisera/media at laboratory for specific purpose. Only answer according to what the laboratory has available ON THE DAY OF THE ASSESSMENT. Following are choices available: o 1: Always available in the laboratory o 2: Available for part of the time (>6 months/year) o 3: Not Available (regular shortage or never available) o 4: Not Applicable (reagents not necessary for the laboratory) If the laboratory regularly stocks a reagent, but does not have it stocked on the day of the assessment, your choice of answer should be « 2 ». Choice « 4 » should be used when the laboratory does not stock certain reagents and the laboratory does not perform tests requiring those reagents. Feel free to use « 4 » in answering questions in this section in order to have proper representation of the indicator (choosing « 3 » instead of « 4 » will have direct impact on the calculation of the indicator) 11- Module 6: tests This is the longest and most important module in LAT. Hence it is recommended to be performed at the last part of assessment. It is imperative to recognize the difference between the two types of « no, I am not able to perform this analysis » for each question in this section. Following are two possible cases: The laboratory does not perform the test because it is irrelevant for the purpose of the laboratory (or the laboratory is not responsible in performing such test). User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 22/52 The laboratory does not perform the test because of the lack of reagents, equipments, or skilled personnel. Overall, the module is categorized in two separate parts: Screening Tests Confirmation Tests Examples in distinguishing two different cases of « no, I am not able to perform this analysis » answer is given below. NOTE: All the questions should be answered based on the availability of the test on the DAY OF THE ASSESSMENT. 1. Screening tests: Example: Diphtheria Screen Test: 1- If screening tests are not necessary for the laboratory or are not part of the laboratory duties Figure 10 : screening test for diphtheria not needed Answer « Y » on the first raw 2- If screening tests are necessary but not performed due to a lack of reagents/stock/equipment Figure 11 : screening test for diphtheria not done o Answer « N » on the first and last row, and « Y » on the second one in order to reflect this condition in LAT. 3- If screening tests are performed Figure 12 : screening test for diphtheria done User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 23/52 o Answer « N » for the first two rows and « Y » on the last row (which indicates specific screening test(s) that is being performed at the laboratory). Note: For other tests, several screening tests may be available. Answer « Y » for each relevant screen tests being performed in the laboratory. Once this section is finished, count the total number of diseases screened at the laboratory and enters this number in the cell « B5 ». Confirmation tests: Answering these questions should be similar to the previous section. However, note the following differences: It is not necessary to count the number of diseases screened in this section. For each test, you must enter the number of tests performed in the past year preceding the assessment. Example 1: Bacillary Dysentery: Figure 13 : confirmation test for dysenteria If confirmation is performed answer « N » for the first two rows. For the rest of the question, answer « Y » or « N » accordingly based on the laboratory to identify specific tasks being performed for the test at the laboratory (ex. culture, tube and gallery (API type), serotyping by agglutination (sd1, flexneri, sonnei …) If the laboratory performs antibiogramme (« AST »), answer « Y » as in the Figure 12. For #### section, fill in the number of bacillary dysentery tests performed at the laboratory in the past year preceding the assessment. Example 2: Tuberculosis (TB) User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 24/52 Figure 14 : confirmation test for tuberculosis not done 1. If the laboratory does not perform any TB tests it may be because of following reasons: 1) The laboratory is too small (intermediate/periphery). 2) TB testing is not the main duty of the laboratory (reference laboratory specializing in diseases other than TB). For these two cases, answer « Y » for the first question and select « N » for the rest of the questions. 2. If lack/shortage of reagents or equipment prevents the laboratory to perform TB test, you should answer « Y » for the second question and « N » for the first and rest of the questions in the section. Otherwise, answer « Y » or « N » based on the laboratory. 12- Module 7: Laboratory staff & working time Laboratory Staff For this section, record the number of staff members in each category (specialty) listed in LAT. Following are the relevant categories: o Senior staff (doctor, biologists, pharmacist biologist, PhD., or other high level scientists) o Full time laboratory technicians o Custodians and janitors or staff in charge of cleaning and sterilizing o Total number of staff including the number counted in the previous three categories, as well as secretaries and stock managers) Staff Training Use following parameters when answering questions in this section. For relevant questions, if the laboratory meets these conditions, answer « Y ». User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 25/52 1) Formal on-site training: A training performed by professionals from outside the laboratory. Official training certificates must be issued following the training. 2) Training at a national laboratory level: a formal training should be carried out by the laboratory responsible or by an external professional group. Again, official training certificates must be issued following the training. 3) International training: Access to a relevant training for one or more staff members at a location outside of the country. 4) Informal on-site training: Continuous internal review and training of the technicians (ex. coproculture, malaria diagnosis, microscopy, etc.). Test Selection For this section, do not give the list of possible answer choices to the respondent. Only provide these choices when the response is significantly different from the three answer choices. Opening days and hours For this section, answer following questions accordingly: o Number of days the laboratory is operates in a week (for a laboratory opened 5 days ½ per week, count as 5 days) o Number of hours the laboratory operates per day (this should be an average) o Number of staff working overtime or outside the normal working hours (usually relevant for laboratories associated with a hospital that has a permanent nightshift positions) o Samples received after hours NOTE: When entering the number of staff working overtime or working outside the normal working hours consider the following: 1) Number of staff members present in the laboratory during the night shifts at a hospital. Or 2) In case of PHL (all the staff members work during normal operating hours), enter only “1” if one laboratory personnel resides in a location less than 30 minutes from the laboratory (to come to the lab in case of emergency). Procedures in handling specimen and samples received outside the office hours) User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 26/52 Figure 15 : critical thinking toward samples during night and week end These questions should only be answered if «The laboratory accepts specimens after working hours? » question is answered « Y ». Again, this is to assess the laboratory’s general procedure and the personnel’s critical thinking when receiving the samples or specimen (CSF, blood, urine, and stool) after laboratory operational hours. Do not give out the possible answer choices unless the answer given by the respondent is significantly different from the choices available. Only choose one answer in « Y » and rest in « N » for the relevant sample/specimen being handled after operational hours of the laboratory. Answer « na » if the sample/specimen is not handled after the operational hours of the laboratory (figure 14.) 13- Module 8: Total quality Analysis procedures Written procedures (printed or handwritten) have to be present in order to answer « Y » at the first question For the two other questions about language and accessibility of the procedure, see page 16Error! Bookmark not defined. For the first question in this section, you must verify whether or not the laboratory being assessed has written analysis procedures (printed or handwritten). If the laboratory is able to show you this document, answer « Y ». Otherwise, answer « N ». User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 27/52 In answering next two questions in this section regarding the language and accessibility, please refer to page 16. Internal quality control (IQC) Question numbers: 1. This question concerns availability of any WRITTEN or PRINTED list of different IQC implemented by the laboratory. In order to answer « Y » for this question, the laboratory must have at least two from the following set: 1) AST control with standardized strains 2) Culture media control 3) IQC for serology (both positive and negative control) 4) IQC for staining methods (Gram and other tests) 2. This question concerns availability of ATTC or other standardized strains. If the laboratory possesses AT LEAST 3 ATCC strains (E. coli, S. aureus & P. aeruginosa) or 3 other standardized strains (Pasteur, CDC, etc.), answer « Y » for this question. 3. This question concerns assessment of the quality of AST at the laboratory. If the laboratory performs WEEKLY assessment of the quality of AST with at least 3 strains, answer « Y ». If with 2 strains and/or monthly, answer « N ». 4. This question concerns the quality of any in-lab made culture media. If the laboratory has an adequate system or procedure of testing sterility for any in-lab made culture media, answer « Y »10. 5. For this question, if the laboratory uses BOTH negative and positive controls for ANY serologic tests (including HIV tests and doctor tests), answer « Y ». If this is not done in one of serologic tests, answer « N ». External Quality control (EQC) For this section, only answer « Y » for both questions (serology and bacteriology) if the laboratory have participated in any EQC program for both serology and bacteriology IN THE PAST 12 MONTHS. You must be able to verify this (by asking for a certification or form). Answer « na » if each question is not relevant to the laboratory (ex. Viral EQC in a bacteriological laboratory ( not applicable). Availability of temperature charts For questions in this section, answer based on what you observed ON THE DAY OF THE ASSESSMENT. Temperature charts must be present in relevant equipment in order to answer « Y » for each question. Preventive maintenance of laboratory equipments User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 28/52 These set of questions are designed to assess how laboratory equipments are maintained. o Answer « Y » for the first question if the laboratory conducts regular preventive maintenance on all of their equipments. o Answer « Y » for the second question if there are written or printed preventive maintenance procedures for each equipment available to the laboratory staff. o Answer « Y » for the third question if the laboratory has a permanent staff member assigned for laboratory equipment maintenance. o Answer « Y » for the fourth question if the laboratory has various contracts with private companies for regular maintenance of their equipments. o Answer « Y » only if each laboratory equipment has a maintenance logbook. The maintenance logbook should be visible and located near or at the equipment. Regular Equipment Adjustments/Calibration Following parameters should be met to answer « Y » for relevant question in this section: o Automatic pipette must be check and calibrated AT LEAST once a year (for accuracy, precision, and lubrication) o Kohler adjustment for microscopes must be performed every three months. o Spectrophotometers and ELISA readers must be checked and calibrated AT LEASE once a year (for parasitic light, linearity, and wavelength). Note: these adjustments can be done by an external company for equipments such as spectrophotometers and ELISA readers. User manuals and spare parts Following must be met in order to answer « Y » for relevant questions: o AT LEAST 75% of all laboratory equipments must have a user manual and these manuals should be in the common language of the country o A basic set of spare parts for each equipment must be available inside the laboratory. Examples for this include: Microscope bulbs Incubator, distillatory or autoclaves resistors (?) A small stock of various fuse A basic tool box Etc. User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 29/52 14- Module 9: Reporting, analysis and communication NOTE: for all yellow celled questions, please refer to section 6 (page 8) before answering. Answer all the relevant questions in Y, N, or na. General reporting Please consider following when answering « Y » to each question in this section: o Efficient communication between disease surveillance services and the laboratory (ex. Minimum weekly update of the list of disease under surveillance diagnosed in the laboratory) o Presence of an updated list of priority disease and/or disease under surveillance (this list should be available to all staff members working in the laboratory and clearly displayed). Guidelines regarding IDSR should also be available INSIDE the laboratory. o Availability of a procedure clearly specifying how disease under surveillance are reported o Availability of a standardized form for disease reporting (a simple duplicable form which can be filled by hand is sufficient enough). o Availability of a standardized form for disease reporting in the document archives. Documentation of general laboratory activities Please consider following when answering « Y » for questions in this section: o A systemic use of laboratory logbook o Documentation of basic analysis performed at the laboratory (ex. Number of samples, number of positively identified samples, distribution of microorganisms, AMR sensitivity profiles, etc.) o Availability of archived monthly summary of the laboratory activities (sent on a monthly basis to the laboratory officials, including the laboratory director and other relevant authorities of the laboratory). Electronic recording of the data NOTE: This section only concerns reference/level 3 laboratories. For other laboratories (intermediate and peripheral level laboratories), answer « na » for each question. Please consider following when answering « Y » for questions in this section: o Availability of electronic archive (in MS Word or MS Excel format) of monthly summary of all laboratory activities. User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 30/52 o Availability of electronic archive (in MS Word, MS Excel, or PDF format) of monthly summary of all laboratory activities to the laboratory officials. o Regular back-up of the laboratory data (on floppy disk, CD, or USB data storage). It is recommended to perform data back-up daily. Sample referrals NOTE: Questions in this section mainly concerned with smaller laboratories (intermediate and peripheral level). However, in some cases, reference laboratories may also refer samples to WHO CC or other international laboratories. Answer « na » in this section if referral of the sample is not relevant for the laboratory. In answering questions in this section, ask the respondent if any samples, including bacteriology samples, are sent to a reference laboratory for analysis. If so, ask if they have special methods (or boxes) for this purpose. Answer accordingly. Supervision of the laboratory In order to answer « Y » to these questions, relevant laboratories must be supervised annually: o By an intermediate or higher level laboratories for a peripheral laboratory o By a reference laboratory for an intermediate laboratory o By an international specialists (WHO, WHO CC labs, or other international laboratories) or collaborative centers for a reference laboratory Laboratory collaborations Please consider following when answering « Y » for questions in this section: o The laboratory has some sort of agreement with laboratories in the region to share necessary reagents/media in case of emergency o The laboratory has a correspondent for communication with supervising party in case of emergency or question: (intermediate and peripheral laboratories) for national level laboratories and (international laboratories and WHO Collaborative Centers) for reference laboratories o The laboratory accepts and manages samples/specimens from other laboratories, if needed. o The laboratory supervises lower level laboratories. 15- Module 10: outbreaks General involvement during an outbreak User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 31/52 Please consider following parameters before answering « Y » for questions in this section: o The laboratory has at least one representative or correspondent for organizing meetings/crisis committee during the epidemic control or in case of an outbreak o The laboratory have analyzed samples from AT LEAST two out breaks or 2 suspicion outbreaks (depends on the laboratory’s capacity level) Sources of supplies during an outbreak Answer in all applicable part in this section. If the question does not apply to the lab (meaning the laboratory did not receive any supply during past 12 months before the assessment from a specific organization) answer « N ». Answer « Y » only if the laboratory received free reagents and other laboratory supplies AT LEASE ONCE from relevant partners or organizations during the past 12 months before today’s assessment. Outbreak participation and investigation Please consider the following guidelines before answering « Y » for questions in this section: o Has one or more laboratory staff (microbiologist or laboratory technician) traveled to an outbreak location or participated in a field study for a purpose of (possible) outbreak investigation in the past 2 years? If so, answer « Y » for the first question. o Has the laboratory been consulted for preparation/shipment/handling of samples by other laboratories? IF so, answer « Y » for the second question. o Did the laboratory ever handle samples referred from other laboratories located in an outbreak region during an outbreak? If so, answer « Y » for the third question. o In case of an outbreak in the region in which the laboratory is responsible, has the laboratory referred its samples/specimens to a reference or other laboratories for further analysis? If so, answer « Y » for the fourth question. Availability of specific guidelines for outbreaks For this section, answer « Y » or « N » for only relevant questions. Feel free to respond « na » if the question does not apply to the laboratory (ex. Plague in Western Africa). If answering « Y », you need to make sure that the laboratory has a specific guideline for each relevant disease (Hemorrhagic fever, Plague, Cholera, Meningitis) OR an IDSR guideline in INSIDE the laboratory. User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 32/52 Specific outbreak procedures Please consider following parameters when answering questions in this section: o Does the laboratory have specific procedures during an outbreak? (This includes procedures for transporting media, organizing intervention teams, and handling other emergencies during an outbreak). If so, answer « Y » first question. o Does the laboratory keep or document all samples received and tested that are linked to an outbreak in a special logbook or in a mark them on a regular logbook? If so, answer « Y » for second question. o Do the laboratory personnel have an easy access to a contact person other relevant staff members in case of an outbreak? IF so, answer « Y » for the third question. Critical thinking with samples in case of outbreak Often, reagents are limiting factor in most laboratories when analyzing a large number of samples. Because these samples may have similar result, this question is designed to show whether or not the laboratory can save precious resources if large number of samples arrive at the laboratory during an outbreak. Similar to previous questions, assessing critical thinking of a laboratory personnel, ask this question to the responding person WITHOUT giving him/her the list of possible answers. If the respondent does not answer or does not know the answer, answer « 1 ». 16- Analysis of the results and summary General overview in analyzing indicator summary When analyzing results of the indicators, it is imperative to do this with the respondent from the laboratory who has been working on this assessment with you. This should be done at the end of the assessment and after entering all the data in LAT. NOTE: Discuss not only the weaknesses, but also the strengths of the laboratory on which an improvement plan can be built After analysis, highlight only the three most important indicators that need to be implemented for the laboratory’s improvement in the future. Figure 16 shows an example of the graphic representation of indicators in LAT with background color ranging from red to green. This allows for you to easily assess conditions of each indicator: Red: Below 50%, requires significant improvement Yellow: Between 50 and 85%, some improvement is necessary User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 33/52 Green: Above 85%, the laboratory is in good standing Figure 16 : sheet containing all indicators and their graphic representation General indicator summary For the summary of general indicator (located in the « Indicator Summary » Sheet), it is possible to choose different modules in calculating the general indicator of the laboratory. To choose specific modules, select « Y » or « N » in the left corner (Figures 17-18). The default value is « Y ». Choosing « N » will exclude that specific module for the final calculation (a simple average). Figure 17: general indicator calculation, including the 10 modules This calculation is an average of all modules or any combination of selected modules. This allows you (the assessor) to figure out which indicator contributes the most to the final summary this assessment. In other words, you can User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 34/52 graphically interpret which modules and how much of the particular module needs to be improved in order to reach recommended overall level. Figure 18: calculation of the general indicator without the modules 2 and 8 For an example, refer to the difference between Figure 18 and 19. In this example assessment, we can find that biosafety and total quality (modules 2 and 8) contribute about 8% decrease in the general indicator Weighed indicators When calculating the overall indicator for each module, LAT is also capable of emphasizing and de-emphasizing the importance of specific indicator and its contribution in the calculation of the overall indicator by means of weight categories. LAT incorporates three weight categories (1: less important, 2: important, 3: very important) which can be selected individually in a special indicator summary page called “weighed indicator” sheet in LAT (Figure 17). Default value is « 2 » (important). 3 2 4 1 Figure 19: weighted indicator sheet User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 35/52 This sheet summarizes the following: un-weighed indicators (1) both un-weighed (2) and weighed (3) module score weighing coefficients (4), « 2 » by default (important) NOTE: in this worksheet, enter ONLY the coefficient value (1, 2 or 3). When clicking on the arrow close to the cell, a list of the 3 coefficients is revealed (figure 20). After entering all the coefficients, the new module summary (3) is automatically calculated. Figure 20: list of available coefficients Weighed general indicator summary It is also possible to configure general indicator summary page to reflect the relative importance of each module in the calculation of the general indicator. Like the previous, there are also three choices (1: less important, 2: important, 3: very important) to reflect the relevant importance of each module in the overall calculation of the general indicator. Hence, the result of general indicator will change according to the weight adjustments for each module (Figure 22). The default value is « 2 ». Please note that the reason for implementing this system is to evaluate the overall indicator more accurately by prioritizing and reflecting the most important modules in analyzing the assessment result. Figure 21: general un-weighed indicator Figure 22: difference in general un-weighed indicator and general weighed indicator. Choosing same weight, for instance « 2 », will produce a result that is no different from the un-weighed module summary (if no coefficient was attributed to each indicator). Similar to the general summary page, you can also choose to User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 36/52 include or exclude specific module in the overall calculation of the general indicator by choosing « Y » or « N » in the left corner. The default value is « Y ». NOTE 1: There is two weighing processes one after the other: For each indicator module summary For each module summary global indicator NOTE 2: Upon the final review of LAT, each indicator will have specific weight contribution to the overall calculation of the general indicator based on its relevant importance. Once these parameters are set, weights for each indicator and module will be locked and protected against changes, especially for the version to be used in the actual laboratory assessment. However, an unlocked version will be available for assessed laboratories and assessor for review and perform additional analysis of the assessment results. 17- Un-protecting & Editing LAT Each worksheet in LAT is protected against any modification. This is to avoid performing wrong manipulation that may compromise calculation. Some « boards » have been placed in certain locations to hid cells that are used for calculations. NOTE: To unprotect these worksheets in LAT, click on “tools” in the menu bar in Excel to find “protection.” Then click “unprotect sheet” as shown in the diagram below (Figure 18). Figure 23 : active sheet unprotected User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 37/52 This will unprotect each worksheet in LAT and you may be able to move the boards to reveal calculation section, add or delete questions, change indicator calculations, or add/delete indicators. CAUTION: In doing this task, first save your work in a different name so that you may go back to the original file. NOTE: your changes and improvements may be an interest to us as this is still a developing project. We kindly ask you to send us your new version or revised version to the following email addresses if possible: piersona@lyon.who.int Figure 24 : right board un-removed Figure 25 : right board removed once unprotected, calculations can be seen User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 38/52 Appendix 1: indicators calculation 1- Building facilities and utility service *** BUIDLING CONDITIONS*** * for the next 3 questions, choose from following: 3-good, 2-medium, 1-bad Condition of the roof Condition of floors Condition of walls # # # Indicator calculation: Good 100% Medium 50% Bad 0% Final indicator = average *** FLUIDS CONDITIONS *** What percentage of working hours are the following available? ELECTRICITY ### RUNNING WATER ### Do you have an emergency electric generator or other back up power source? <Y> Do you have a safe sewage disposal method? <Y> *** % OF BENCHED ROOM UTILIZED*** Rooms in the building ### Number of laboratory rooms in current usage ### Indicator calculation: 2 first questions % last one, yes = 100%, no = 0% Final indicator = average Indicator calculation: % of room utilized first indicator = # of benched rooms utilized/ # of benched rooms available. *** NUMBER OF BENCHED ROOMS (LEVEL DPT.)*** Number of rooms with laboratory benches ### Number of laboratory rooms in current usage ### Indicator calculation: Level 100% 50% >9 >6 >6 >4 >3 >2 * Final indicator = Average 1 2 3 *** COMMUNICATION (ACCESS TO INTERNET)*** Telephone <Y> Fax <Y> E-mail <Y> Regular mail service <Y> Computer (inside the laboratory) <Y> *** COMMUNICABLE DIEASE COVERAGE *** Does the Laboratory perform following: Bacteriology microscopy? <Y> 0% <6 <4 <2 Indicator calculation: Final indicator = average Indicator calculation Calculation of normal workload, based on the level of the laboratory User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 39/52 Bacteriology culture? <Y> Serological analysis? <Y> Virology culture? <Y> Parasitology analysis? <Y> Mycology microscopy and/or culture? <Y> Molecular biology analysis? <Y> Tuberculosis microscopy <Y> Tuberculosis culture <Y> level 3: all (total 9) level 2: all except virology, molecular biology (total 6) level 1: bacteriology microscopy, parasitology, mycology, and TB bacilloscopy (total 4) 2- Biosafety, hygiene and security *** USE OF SAFETY EQUIPMENT *** Does the laboratory personnel/staff use following while working in the laboratory? *choose from following: 1 never, 2 sometimes, 3 always Labcoat? Gloves? Protective masks? Protective glasses? *Where are labcoats and laboratory linens washed? 1 home, 2 central lab, 3 outside laundry service # # # # # *** AVAILABILITY OF SAFETY PROCEDURES *** For hand washing? <Y> For disinfection of contaminated materials? <Y> For sterilization? <Y> For glassware and equipment washing? <Y> For waste disposal? <Y> For laboratory cleaning? <Y> For laboratory related injury? <Y> For fire emergency? <Y> For protection against specific infectious agents? <Y> Indicator calculation always 100% sometimes 50% never 0% for clothes washing: home 0% other 100% Final indicator = average Indicator calculation Final indicator = average *** LEVEL OF SAFETY TRAININGS *** Security while manipulating laboratory samples <Y> Classification of different classes of micro-organisms <Y> Security while sampling <Y> Methods in packaging and triple-packaging of a sample <Y> Using disinfectants and procedures in disinfections <Y> Proper waste management (handling hazardous and non hazardous wastes) <Y> Indicator calculation: Final indicator Average *** SAFETY CONDITIONS *** Indicator calculation Do you regularly use liquid disinfectant (incl. HYPOCHLORITE) when disinfecting the laboratory? <Y> Do you have a sink for washing hands only? <Y> Are the benches solvents and chemicals resistant? <Y> Are the benches easily washable? <Y> Final indicator = average User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 40/52 *** DISINFECTION/STERILIZATION OF EQUIPMENTS *** Do you regularly disinfect your centrifuge? <Y> Do you regularly disinfect your incubators? <Y> Do you always use temperature strips for sterilization? <Y> Indicator calculation Final indicator = average *** WASTE DISPOSALS *** Do you have separate disposals for infectious and non-infectious wastes? <Y> Do you have covered waste disposal containers? <Y> Do you have safe waste containers <Y> Do you have special sharps containers <Y> Do you have dedicated waste for used solvents <Y> Indicator calculation Final indicator = average *** AVAILABILITY OF BIOSAFETY DOCUMENTATION *** Do you follow specific biosafety guideline? <Y> Do you have the WHO laboratory biosafety manual (3rd edition)? <Y> Indicator calculation Final indicator = average 3-Specimen collection, handling and transportation *** QUALITY OF SAMPLES RECEIVED *** Do you encounter following problems when receiving samples? Answer by: (3) never, (2) sometimes or (1) always? WRONG PACKAGE <A> WRONG CONSERVATION <A> SAMPLES IN INADEQUATE MEDIA <A> WRONG IDENTIFICATION <A> DELAY IN SAMPLE DELIVERY <A> *** SAMPLING PROCEDURES *** Stool sampling? <Y> Standard procedures for blood sampling? <Y> CSF sampling? <Y> Throat sampling? <Y> Are the procedures written in the most appropriate language? <Y> Does the staff have an easy access to these procedures <Y> Are these procedures available to clinicians and non-laboratory staff collecting the samples?<Y> *** QUALITY OF SAMPLING REQUEST FORM *** Does the lab provide standard request forms to order lab test? <Y> * If yes, does the form contain the following: DATE of sample? <Y> TIME of sample? <Y> NAME of the patient? <Y> AGE of the patient? <Y> SEX of the patient? <Y> ADDRESS of the patient? <Y> TYPE OF SPECIMEN of the patient? <Y> TEMPERATURE of the patient? <Y> CLINICAL DETAILS of the patient? <Y> NAME OF THE SUBMITTING CLINICIAN? <Y> User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 Indicator calculation always 0% sometimes 50% never 100% Final indicator = average Indicator calculation Final indicator = average Indicator calculation: If response to first question = no indicator = 0 Otherwise, final indicator = average 41/52 *** CRITICAL THINKING ABOUT SAMPLES *** What do you do if the requested information is not complete? <A> * 1 (TEST SAMPLE ANYWAY WITHOUT CONTACTING ANYBODY) * 2 (NO ANALYSIS IS PERFORMED AND SAMPLE IS REJECTED) * 3 (TRY TO CONTACT THE SENDER OR THE PATIENT) Indicator calculation 1 and 3 0% 2 100% *** QUALITY OF THE LOGBOOK *** Do you have an unique identification number or system for locating specimen? <Y> If YES, do you record by the NAME of the patient? <Y> by the AGE of the patient? <Y> by the SEX of the patient? <Y> by the ADDRESS of the patient? <Y> by the TEMPERATURE and/or the CLINICAL DETAILS of the patient? <Y> by the NAME OF THE PRESCRIBER of the patient? <Y> by the TYPE of specimen? <Y> by the DATE of sampling? <Y> Indicator calculation Final indicator = average *** MACROSCOPIC EXAMINATION *** Do you perform macroscopic examination of the samples? <Y> Are the macroscopic examination results of the sample recorded? <Y> Indicator calculation Final indicator = average *** SPECIMEN STORAGE *** What do you usually do with the specimen after testing <A> * 1 (IMMEDIATE DESTRUCTION) * 2 (KEEP 3-5 DAYS AND DESTRUCTION) * 3 (OTHER) Indicator calculation 1 = 0% 2 = 100% 3 = 100% *** QUALITY OF SPECIMEN TRACKING *** Do you keep track of the specimen or samples (specimen) received? <Y> Are you able to easily find a previous result? <Y> * if yes, can you find the records of a specimen by: Can you find it using the patient name? <Y> Can you find it using the sampling date? <Y> Can you find it using the results? <Y> Indicator calculation Final indicator = average 4-Equipment *** 1) MINIMAL FUNCT. EQUIP. 2) OPTIMAL FUNCT. EQUIP. 3) BASIC MINIMAL FUNCT. EQUIP.T 4) BASIC OPTIMAL FUNCT EQUIP. *** autoclave basic scale Indicator calculation: (level dpt.) in % available At the beginning of the assessment, a national consensus on equipment by level has to be reached User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 42/52 binocular microscope candle jar CO2 incubator computer + printer centrifuge electrophoresis equipment ELISA equipment (W/I/R) fluorescence microscope freezer-20° freezer-70° fridge gel pulse electrophoresis glassware kit * heated magnetic agitator incubator, large incubator, small Internet connection (year) manipulation box McFarland photometer media dispenser oven pH meter automatic pipettes Plexiglas screen precision scale (electronic) rotative agitator safety cabinet class II safety cabinet class III thermocycler vortex distiller waterbath first. The indicators will be then calculated depending on this list The basic equipment indicators will be calculated in the same way but only these equipments will be included in the calculation: autoclave basic scale binocular microscope candle jar ELISA equipment (W/I/R) freezer-20° fridge incubator (any types) water distiller waterbath Glassware will be calculated based on following ratings: 0- Don’t have any laboratory glassware 1- Shortage (laboratory glassware is available, but not enough to cope with the capacity of the laboratory). 2- Minimum (the laboratory has just enough glassware for normal operation). 3- Optimal (the laboratory has more than enough glassware for its capacity). 5-Reagents and supply *** REAGENT PREPARATION FROM POWDER *** Do you prepare reagents in the laboratory? <Y> Indicator calculation Final indicator = average of answers from Gram, blood agar, Mc Conkey or BCP media, and ‘quality control’ questions. *if YES, are the following media or reagents prepared from row material or ready-to-use powders? Gram staining <Y> Blood agar <Y> Mc Conkey or BCP media <Y> *if YES, do you quality control these home made reagents? <Y> *** QUALITY OF REAGENT MANAGEMENT *** Do you have stock cards for your reagents <Y> Do you manage your stock electronically <Y> Do you check regularly expiration dates of your reagents Do you write the date of opening written on the reagents Do you perform at least 2 yearly inventories of your stock Indicator calculation Final indicator = average <Y> <Y> <Y> User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 43/52 *** AVAILABILITY OF FUNDS FOR REAGENTS *** Do you have dedicated funds for your laboratory Do you receive funds from MOH? Do you receive funds from Public Health Authorities Do you receive funds from cost-recovering Do you receive funds from international cooperation? *** USE OF EXPIRED REAGENTS *** Do you use expired products and reagents? (0 = regularly, 1 = sometimes, 2 = never) <Y> <Y> <Y> <Y> <Y> Indicator calculation Final indicator = average Indicator calculation 0 0% 1 50% 2 100% # For all the next set of questions, following are calculation parameters: *1: *2: *3: *4: always 100 % sometimes (AT LEAST 6 MONTHS PER YEAR) 50% enteric never, 0% not applicable (not needed) does not enter in the calculation *** AVAILABILITY OF BASIC STAINING REAGENTS *** Gram staining # Methylen blue staining # Giemsa staining # May Grünewald Giemsa or field staining # Ziehl staining # Lugol staining (iodine/iodure) # Indicator calculation *** AVAILABILITY OF SPECIAL STAINING REAGENTS *** Trichrome staining Lactophenol blue staining Weber staining China ink contrast (CSF) Gomori Grocott staining Indicator calculation # # # # # Final indicator = average Final indicator = average *** AVAILABILITY OF ENTERIC TRANSPORT AND CULTURE MEDIA *** Cary-Blair transport media # Alkaline peptone water transport media # Hektoen or other Salmonella-Shigella culture media # Mc Conkey culture media # TCBS culture media # *** MENINGITIS TRANSPORT AND CULTURE MEDIA *** TransIsolate transport media # Blood agar culture media # Chocolate culture media # *** AVAILABILITY OF OTHER TRANSPORT AND CULTURE MEDIA *** Indicator calculation Final indicator = average Indicator calculation Final indicator = average Indicator calculation User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 44/52 Sabouraud culture media Chapman culture media Hemoculture bottle Löwenstein-Jensen culture media Brain heart broth or equivalent broth # # # # # Final indicator = average *** AST REAGENTS AND CULTURE MEDIA *** Muller Hinton 2 culture media # Plastic Petri dish for AST # Antibiotic disks for AST # Indicator calculation Final indicator = average *** AVAILABILITY OF SPECIFIC ANTISERA *** Neisseria meningitidis # Salmonella spp. # Shigella spp. # Vibrio spp. # Staphylococcus spp. # Streptococcus spp. # Escherichia coli O157 # Indicator calculation Final indicator = average *** AVAILABILITY OF SEROLOGY REAGENTS *** Hepatitis A # Hepatitis B # Hepatitis C # HIV # Measles # Yellow fever # Viral hemorrhagic fever # Rubella # Indicator calculation Note: not done for level 1 Final Indicator = average 6-Analysis and Test performed *** AVAILABILITY OF SCREENING FOR TARGETED DISEASES AVAILABILITY OF REAGENTS FOR SCREENING TESTS *** *Screening tests for diphtheria Non applicable <Y> Possible to do, but not done <Y> Sampling and Gram-staining <Y> Indicator calculation *Screening tests for hepatitis B Non applicable Possible to do, but not done Transaminasis Other liver enzymes For each disease: if the answer of ANY of the screening test is “yes”, then the result for the disease is 100% if NO screening test is available, the result for the disease is 0% If the answer to any of the screening tests (disease is not screened by the laboratory) is “na”, the answer will not be part of the Indicator calculation. *Screening tests for tuberculosis Non applicable Possible to do, but not done Bacilloscopy on sputum (ZN) Bacilloscopy on sputum (Auramin) Bacilloscopy on gastric fluids <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> FIRST INDICATOR This indicator will check the availability of laboratories to perform screening tests. User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 45/52 Bacilloscopy on urine <Y> *Screening tests for dysentery Non applicable Possible to do, but not done Sampling and Gram-staining Blood in stool checking <Y> <Y> <Y> <Y> *Screening tests for w. diarrhoea Non applicable Possible to do, but not done Sampling and Gram-staining <Y> <Y> <Y> *Screening tests for meningitis Non applicable Possible to do, but not done Sampling and Gram-staining Proteins & glucose dosage WBC counting & differentiation <Y> <Y> <Y> <Y> <Y> *Screening tests for malaria Non applicable Possible to do, but not done Sampling & Giemsa staining <Y> <Y> <Y> *Screening tests for leishmaniosis Non applicable Possible to do, but not done Sampling the lesion and Giemsa Staining Sampling bone narrow and Giemsa staining *Screening tests for VHF Non applicable Basic coagulation tests (thromb, Quick ...) *Screening tests for brucella Non applicable Sampling and Gram-staining *Screening tests for plague Non applicable Sampling and Gram-staining <Y> <Y> <Y> <Y> Final indicator = average of all “Y” or “N” answered questions. Targeted diseases: Diphtheria hepatitis B tuberculosis dysentery w. diarrhea meningitis malaria leishmaniosis VHF plague If the disease is not a common disease in the assessed country, it will not count towards the calculation of the indicator (ex: malaria in Belarus) If the answer to any of the screening tests (disease is not screened by the laboratory) is “na”, the answer will not be part of the Indicator calculation. SECOND INDICATOR % of responses “Possible to do, but not done” that will give an idea of the availabilities of reagents (for both screening AND confirmation tests) on THE DAY OF THE ASSESSMENT <Y> <Y> <Y> <Y> <Y> <Y> *** AST AVAILABILITY AVAILABILITY OF IDENTIFICATION AVAILABILITY OF HIGH LEVEL IDENTIFICATION AVAILABILITY OF VERY SPECIFIC TESTS *** Indicator calculation *Confirmation tests for diphtheria Non applicable Possible to do, but not done Culture and tube identification Culture and gallery identification AST for diphtheria Note: if N/A, it doesn’t count for the indicator (so, lowest level will not be penalized) <Y> <Y> <Y> <Y> <Y> AST availability diphtheria, dysentery, watery User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 46/52 Toxin checking *Confirmation tests for measles Non applicable Possible to do, but not done IgM serology on microplates IgM automated immunoenzymatic serology IgM radioimmunology serology <Y> <Y> <Y> <Y> <Y> <Y> *Confirmation tests for hepatitis B Non applicable <Y> Possible to do, but not done <Y> HbS Ag serology on microplates <Y> HbS Ag automated immunoenzymatic serology <Y> HbS Ag radioimmunology serology <Y> Other Ag are checked <Y> *Confirmation tests for rubella Non applicable Possible to do, but not done IgM serology on microplates IgM automated immunoenzymatic serology IgM radioimmunology serology *Confirmation tests for tuberculosis Non applicable Possible to do, but not done Culture on Löwenstein-Jensen media Culture on Coletsos media Culture on other media Diagnosis by PCR Direct AST on solid media Indirect AST on solid media AST on liquid media *Confirmation tests for dysentery Non applicable Possible to do, but not done Culture and tube identification Culture and gallery identification Immunotyping by agglutination AST *Confirmation tests for w. diarrhoea Non applicable Possible to do, but not done Culture and tube identification Culture and gallery identification Immunotyping by agglutination AST *Confirmation tests for meningitis Non applicable Possible to do, but not done <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> diarrhea, plague, meningitis, brucella yes = 100%, no = 0%, indicator = average indicator on identification: diphtheria identification is OK if tube OR gallery is OK measles identification is OK if any IgM is OK B hepatitis identification is OK if any Ag HbS is OK Rubella identification is OK if any IgM is OK TB culture identification (all media) Dysentery identification is OK if tube OK gallery is OK Watery diarrhea identification is OK if tube OR gallery is OK Plague serology (F1) Meningitis identification is OK if tube OK gallery is OK Brucella basic agglutination (ASW & rose bengale OK) indicator on high level identification : gallery ID diphtheria Measles immunoenzymatic hep B immunoenzymatic or RIA rubella immunoenzymatic or RIA Plague culture + ID Gallery ID meningitis Gallery ID dysenteriae Gallery ID watery diarrhoea Immunological techniques for malaria Leishmania serology VHF serology Brucella serology indicator on “specific tests” diphtheria toxin other Ag for B hep TB PCR TB AST (whatever could be the technique) Dysentery typing Watery diarrhoea typing Bacterial meningitis agglutination Viral meningitis identification Cell culture in virology Antimalaria resistance techniques User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 47/52 Culture and tube identification Culture and gallery identification Immunotyping by agglutination (incl. W135) AST <Y> Viral meningitis diagnosis by agglutination Viral meningitis diagnosis by cell culture <Y> <Y> <Y> <Y> <Y> *Confirmation tests for malaria Non applicable <Y> Possible to do, but not done <Y> Sampling & immuno. techniques (QBC or other) <Y> Antimalaric resistance testing <Y> *Confirmation tests for leishmaniosis Non applicable Possible to do, but not done Bone narrow culture IFI serology *Confirmation tests for VHF Non applicable Possible to do, but not done Serology on microplates Automated immunoenzymatic serology Radioimmunology serology *Confirmation tests for brucella Non applicable Possible to do, but not done Culture and identification Serology on tube (ASW) Rose-Bengal serology IFI serology ELISA serology AST *Confirmation tests for plague Non applicable Possible to do, but not done Serology Culture and tube identification Culture and gallery identification AST <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> 7-Laboratory staff & working time *** PRESENCE OF A SENIOR STAFF % OF SENIOR STAFF PRESENCE OF CLEANING STAFF *** Indicator calculation For all communicable disease units: How many high level laboratory staffs do you have working in your lab? ### PRESENCE OF A SENIOR STAFF nb senior staff >0 % OF SENIOR STAFF User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 48/52 How many laboratory technician do you have working in your lab? ### How many janitors (cleaning staff) do you have working in your lab? ### What is the total of your staff ### *** STAFF TRAINING *** AVAILABILITY OF STAFF TRAINING AVAILABILITY OF FORMAL TRAINING *in the past three years has the laboratory conducted the following: A formal training on site? <Y> A formal training at the national laboratory? <Y> A formal training at international lab? <Y> Any informal training on site? <Y> if > 15 % 100% if 5-15% 50% if <5% 0% PRESENCE OF CLEANING STAFF nb cleaning staff >0 Final indicator = average Indicator calculation any “yes” 100% any “yes” at the 3 first questions 100 % other way 0% Final indicator = average *** ANALYSIS DECISION *** Indicator calculation for the next 3 questions: 1 prescriber, 2 lab responsible (director or high level staff), 3 technician, 4 other Who decides which tests to perform for the samples # Who makes decisions for further tests # Who reviews the results before to sent to the clinicians # If the response is “prescriber” or “responsible” 100% Other way 0% Final indicator = average *** WORKING HOURS AND DAYS OF WORK *** How many days per week do the laboratory staff required to work? # How many hours does the laboratory operate per day? ## On average, how many laboratory staffs work overtime? ## Does the laboratory accept specimen after regular opening hours? <Y> Indicator calculation >= 6 days 100%, 5 days 50% >= 12 hours 100%, 10 hours 50% >0 100% “yes” = 100% Final indicator = average *** CRITICAL THINKING OUTSIDE WORKING HOURS *** *Answer the questions below concerning specimen handling (Answer all based on the type of samples arriving beyond regular hours). Answer Y or N based on how the sample is handed. *** CSF *** Are CSF samples plated immediately? or stored at 4C or maintained at ambient temperature or stored at 35C *** BLOOD*** Are BLOOD samples plated immediately? or stored at 4C or maintained at ambient temperature or stored at 35C <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 Indicator calculation CSF HANDLING 100% if plated, 50% if at 35° BLOOD HANDLING 100% if plated, 50% if at 35° URINE HANDLING 100% if plated, 50% if at 4° STOOL HANDLING 100% if plated, 50% if at 4° other responses = 0% Final indicator = average 49/52 *** URINE *** Are urine samples plated immediately? or stored at 4C or maintained at ambient temperature or stored at 35C *** STOOL*** Are the stools plated immediately? or stored at 4C or maintained at ambient temperature or stored at 35C <Y> <Y> <Y> <Y> <Y> <Y> <Y> <Y> 8-Total quality *** AVAILABILITY OF TECHNICAL PROCEDURES *** Do you have any written standard protocols or guidelines for sample analysis procedures <Y> Are procedures written in a language everybody at the laboratory can easily understand? <Y> Do the staff members have an easy access to these procedures <Y> *** AVAILABILITY OF IQC *** Do you have a clear policy and procedures for performing IQC? <Y> Do you use registered strain (ex. From ATCC) for IQC? <Y> Do you check the quality of AT LEAST 3 AST strains weekly? <Y> Do you perform sterility tests for every batch of the lab made culture media? <Y> Do you use positive/negative control for EVERY serology tests? <Y> Indicator calculation Final indicator = average Indicator calculation Final indicator = average *** AVILABILITY OF EQC *** Has the laboratory participated in any EQC program (for at least two consecutive years)? in bacteriology? <Y> in serology? <Y> Indicator calculation Final indicator = Average of answers from bacteriology and serology. ***AVAILABILITY OF TEMPERATURE CHARTS *** Do you have temperature chart for fridge <Y> Do you have temperature chart for freezers <Y> Do you have temperature chart for incubators <Y> Do you have temperature chart for autoclave <Y> Indicator calculation Final indicator = average *** PERFORMING OF PREVENTIVE MAINTENANCE *** Do you perform preventive maintenance of your equipments? <Y> Do you have written SOP's for each equipment? <Y> Do you have a staff in charge to repair equipments? <Y> Do you have a contract with a private company for preventive maintenance? <Y> Do you have a maintenance log book for your equipments? <Y> *** PERFORMING OF EQUIPMENT ADJUSTMENTS *** * Do you (or an external company) regularly check : automatic pipettes (accuracy, precision, repeatability)? <Y> Kohler centering of your microscope? <Y> Spectrophotometer / ELISA reader? <Y> User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 Indicator calculation Final indicator = average Indicator calculation Final indicator = average 50/52 *** AVAILABILITY OF DOCUMENTATION AND SPARE PARTS *** Do you have user manual for all of your equipments? <Y> Are the manuals available in the common language used at the laboratory? <Y> Do you have a small stock of spare parts (lamps, fuse, filters ...)? <Y> Indicator calculation Final indicator = average 9-Reporting, analysis & communication *** AVAILABILITY OF DISEASE REPORTING *** Do you report the infectious diseases you have diagnosed to other institutions? <Y> Is the list of the diseases you must report available in the laboratory for the staff? <Y> Is there a document or protocol regulating reporting procedures? <Y> Is there a standardized form to report lab results? <Y> Do you keep a record of these notification <Y> Indicator calculation Final indicator = average *** AVAILABILITY OF ACTIVITY RECORDING *** Do you record all the activities of your laboratory in a logbook? <Y> Do you perform basic analysis of your data? <Y> Do you prepare monthly summary report? <Y> Do you send/discuss these reports to/with the authorities? <Y> Indicator calculation Final indicator = average *** AVAILABILITY OF ELECTRONIC ACTIVITY RECORDING *** Do you record all the activities of your laboratory electronically? <Y> Do you prepare reports in an electronic format? (PDF, doc, etc.) <Y> Do you regularly backup of your electronic data? <Y> Indicator calculation *** AVAILABILITY OF SAMPLE REFERRING *** Does your laboratory refer bacteriology or other samples to a reference labs? <Y> Do you have special boxes and procedures specifically for referring samples? <Y> When you refer a sample, do you receive the result back? <Y> *** LABORATORY SUPERVISION *** Are you supervised by other labs or other external companies (at least yearly)? <Y> When supervised, do you always receive the report after each supervision? <Y> Indicator calculation Final indicator = average Indicator calculation Final indicator = average *** AVAILABILITY OF LAB/LAB COLLABORATION *** Do you exchange reagents with other lab in case of shortage? <Y> Do you receive some advice or support from an other lab in case of problems? <Y> Does your laboratory receive samples from other laboratories? <Y> Do you regularly supervise other laboratories? <Y> User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 ONLY for level 1 and 2 Final indicator = average Indicator calculation Final indicator = average 51/52 10- Outbreak participation *** INVOLVEMENT DURING OUTBREAKS *** Is the lab. part of the crisis committee for epidemic preparedness & resp. <Y> Did you participate in any outbreak investigations during the past year? <Y> Indicator calculation Final indicator = average *** SPECIFIC OUTBREAK SUPPLY *** Do you receive (specific) emergency supplies in case of outbreak? <Y> from your government <Y> from NGOs <Y> from WHO <Y> from other international agencies <Y> Indicator calculation Final indicator = average *** OUTBREAK PARTICIPATION *** Do you participate in field investigation? <Y> Do you give advice on how to collect specimens to other labs?<Y> Do you receive specimens from the field during an outbreak investigation? <Y> Do you prepare and send specimens to reference laboratories? <Y> *** SPECIFIC OUTBREAK GUIDELINES *** Do you have IDSR guide for HAEMORRAGIC FEVER?<Y> Do you have IDSR guideline for PLAGUE? <Y> Do you have IDSR guideline for CHOLERA? <Y> Do you have IDSR guideline for MENINGITIS? <Y> Indicator calculation Note: only relevant for African labs Final indicator = average *** SPECIFIC OUTBREAK PROCEDURES *** Do you have a specific procedure in case of outbreak? <Y> Do you have a specific record logbook for outbreak investigation? <Y> Do you know who you have to informed if you diagnose a specific disease?<Y> *** CRITICAL THINKING WITH OUTBREAK SPECIMENS *** * If you receive 50 similar stool specimens from the same area, what do you do? <A> *1 Test them all *2 randomly choose 10 for testing *3 Test the first 10 specimens Indicator calculation Final indicator = average Indicator calculation Final indicator = average Indicator calculation 2 = 100% 3 = 50% 1= 0% User Manual, Laboratory Assessment Tool, WHO/CSR/Lyon, August 2004 52/52