UCH_COMIRB Comparison Tool_Research vs QI vs Program Eval
advertisement
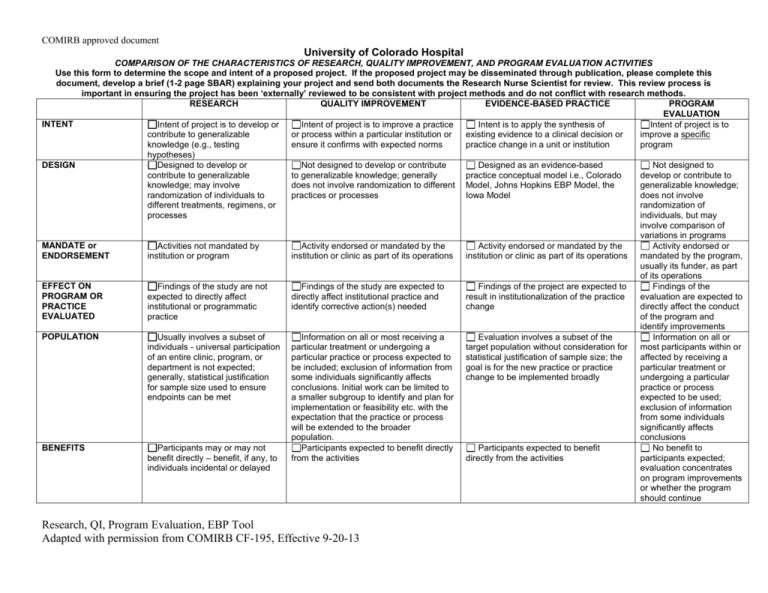
COMIRB approved document University of Colorado Hospital COMPARISON OF THE CHARACTERISTICS OF RESEARCH, QUALITY IMPROVEMENT, AND PROGRAM EVALUATION ACTIVITIES Use this form to determine the scope and intent of a proposed project. If the proposed project may be disseminated through publication, please complete this document, develop a brief (1-2 page SBAR) explaining your project and send both documents the Research Nurse Scientist for review. This review process is important in ensuring the project has been ‘externally’ reviewed to be consistent with project methods and do not conflict with research methods. RESEARCH QUALITY IMPROVEMENT EVIDENCE-BASED PRACTICE PROGRAM EVALUATION INTENT Intent of project is to develop or Intent of project is to improve a practice Intent is to apply the synthesis of Intent of project is to contribute to generalizable or process within a particular institution or existing evidence to a clinical decision or improve a specific knowledge (e.g., testing ensure it confirms with expected norms practice change in a unit or institution program hypotheses) DESIGN Designed to develop or Not designed to develop or contribute Designed as an evidence-based Not designed to contribute to generalizable to generalizable knowledge; generally practice conceptual model i.e., Colorado develop or contribute to knowledge; may involve does not involve randomization to different Model, Johns Hopkins EBP Model, the generalizable knowledge; randomization of individuals to practices or processes Iowa Model does not involve different treatments, regimens, or randomization of processes individuals, but may involve comparison of variations in programs MANDATE or Activities not mandated by Activity endorsed or mandated by the Activity endorsed or mandated by the Activity endorsed or ENDORSEMENT institution or program institution or clinic as part of its operations institution or clinic as part of its operations mandated by the program, usually its funder, as part of its operations EFFECT ON Findings of the study are not Findings of the study are expected to Findings of the project are expected to Findings of the PROGRAM OR expected to directly affect directly affect institutional practice and result in institutionalization of the practice evaluation are expected to PRACTICE institutional or programmatic identify corrective action(s) needed change directly affect the conduct EVALUATED practice of the program and identify improvements POPULATION Usually involves a subset of Information on all or most receiving a Evaluation involves a subset of the Information on all or individuals - universal participation particular treatment or undergoing a target population without consideration for most participants within or of an entire clinic, program, or particular practice or process expected to statistical justification of sample size; the affected by receiving a department is not expected; be included; exclusion of information from goal is for the new practice or practice particular treatment or generally, statistical justification some individuals significantly affects change to be implemented broadly undergoing a particular for sample size used to ensure conclusions. Initial work can be limited to practice or process endpoints can be met a smaller subgroup to identify and plan for expected to be used; implementation or feasibility etc. with the exclusion of information expectation that the practice or process from some individuals will be extended to the broader significantly affects population. conclusions BENEFITS Participants may or may not Participants expected to benefit directly Participants expected to benefit No benefit to benefit directly – benefit, if any, to from the activities directly from the activities participants expected; individuals incidental or delayed evaluation concentrates on program improvements or whether the program should continue Research, QI, Program Evaluation, EBP Tool Adapted with permission from COMIRB CF-195, Effective 9-20-13 COMIRB approved document DISSEMINATION OF RESULTS Intent to publish or present generally presumed at the outset of project as part of professional expectations, obligations; dissemination of information usually occurs in research/scientific publications or other research/scientific fora; results expected to develop or contribute to generalizable knowledge by filling a gap in scientific knowledge or supporting, refining, or refuting results from other research studies Dissemination of information may occur in quality improvement publications/fora; when published or presented to a wider audience, the intent is to suggest potentially effective models, strategies, assessment tools or provide benchmarks or base rates rather than to develop or contribute to generalizable knowledge. Any publication should footnote that the project was carried out as QA and did not meet the definition of research per DHHS regs. CLINICAL SETTINGS (where will project occur, list unit/clinics, etc) USE OF PLACEBO Use of placebo may be planned Comparison of standard treatments, practices, techniques, processes – placebo would NOT be used DEVIATION FROM May involve significant Unlikely to involve significant deviation STANDARD deviation from standard practice from standard practice PRACTICE Dissemination of results can occur in scientific or quality improvement publications and/or presentations; resulting practice guidelines may also be shared Intent to publish or present generally presumed at the outset of the project; dissemination of information to program stakeholders and participants; may be publicly posted (e.g., website) to ensure transparency of results; when published or presented to a wider audience, the intent is to suggest potentially effective models, strategies, assessment tools or provide benchmarks or base rates rather than to develop or contribute to generalizable knowledge. Limitations of publication: any publication should footnote that the project was carried out as QA and did not meet the definition of research per DHHS regulations. Use of comparison group data before practice change (usually current practice). Placebo would NOT be used Program may involve significant deviation from current practice to incorporate current evidence for practice If any of the boxes in the research column are checked then the project must be submitted to COMIRB for review and approval. If the tool indicates that this is quality improvement (QI) or program evaluation (PE) only the signatures detailed below should be obtained Acknowledgment I have appropriately used this tool to evaluate my project entitled: Research, QI, Program Evaluation, EBP Tool Adapted with permission from COMIRB CF-195, Effective 9-20-13 COMIRB approved document By my signature below, I affirm that this project meets the definition of: [Circle the appropriate project description] Research Quality Improvement Evidence-Based Practice Program Evaluation I certify that I will conduct my project in compliance with all federal, state and local laws and policies. If during the course of the project it is amended in such a way as to meet the definition of human subject research under 45 CFR 46 or 21 CFR 56 then I understand that I must submit to COMIRB for review prior to continuing the project. Signature of Project Lead Date Signature of Mentor (if applicable) Date I have reviewed this project proposal and determine that it meets the criteria for quality improvement or program evaluation as outlined above and is an appropriate project to be conducted within this Division/ Department/ School/. e-signature: JoAnn DelMonte, RN-BC, MSN Signature of Director of Professional Resources Or Appropriate Authority (or their designee) Date JoAnn DelMonte, RN-BC, MSN Director of Professional Resources Print Name of Director of Professional Resources OR Appropriate Authority (or their designee) Position Research, QI, Program Evaluation, EBP Tool Adapted with permission from COMIRB CF-195, Effective 9-20-13 COMIRB approved document Guidelines: This document should be retained with the project file and referenced as needed. If you have any questions or concerns that the project may include a research component, submit to COMIRB using the appropriate forms available on the website: COMIRB: http://www.ucdenver.edu/academics/research/AboutUs/comirb/Pages/comirb-home.aspx Definitions: Human Subjects Research For the purposes of this policy “human subject research” is defined as an activity that meets the definition of “research” and involves “human subjects” as defined either by the Common Rule or by FDA regulations. Research definition: A systematic investigation, including development, testing and evaluation, designed to develop or contribute to generalizable knowledge. Activities that meet this definition may be funded or unfunded, or may be conducted as a component of another program not usually considered research. For example, demonstration and service programs may include evaluation components, and may constitute research activities under this definition. Example of Research: Research findings discussed at a unit journal club indicated that applying heat to the extremity facilitated IV catheter insertion. Nurses participating in the journal club questioned whether moist or dry heat was more effective. No literature was found addressing this question. The nurses consulted a Research Nurse Scientist at their organization for help designing a clinical study. A randomized controlled trial was designed to test which form of heat was more efficacious before IV cannulation. A research proposal was developed, IRB approval was obtained, and a grant application was submitted to Sigma Theta Tau Research committee and the team was awarded funding to conduct the study. The study was conducted in the oncology infusion center. It was found that dry heat was the best type of heat with respect to time to cannulation, number of cannulation attempts, and patient satisfaction. The study was presented at a local research symposium and published in the Oncology Nurses Forum. Additional Guidelines for Research: Research, QI, Program Evaluation, EBP Tool Adapted with permission from COMIRB CF-195, Effective 9-20-13 COMIRB approved document For the purposes of this policy, a “systematic investigation” is an activity that involves a prospective study plan which incorporates data collection, both quantitative and qualitative, and data analysis to answer a study question. Investigations designed to develop or contribute to generalizable knowledge are those designed to draw general conclusions (i.e., knowledge gained from a study may be applied to populations outside of the specific study population), inform policy, or generalize findings. Research as defined by FDA regulations means any experiment that involves a test article and one or more human subjects, and that either must meet the requirements for prior submission to the Food and Drug Administration under section 505(i) or 520(g) of the Federal Food, Drug, and Cosmetic Act, or need not meet the requirements for prior submission to the Food and Drug Administration under these sections of the Federal Food, Drug, and Cosmetic Act, but the results of which are intended to be later submitted to, or held for inspection by, the Food and Drug Administration as part of an application for a research or marketing permit. The terms research, clinical research, clinical study, study, and clinical investigation are synonymous for purposes of FDA regulations. [21 CFR 50.3(c), 21 CFR 56.102(c)] Experiments that must meet the requirements for prior submission to the Food and Drug Administration under section 505(i) of the Federal Food, Drug, and Cosmetic Act means any use of a drug other than the use of an approved drug in the course of medical practice. [21 CFR 3 12.3(b)] Experiments that must meet the requirements for prior submission to the Food and Drug Administration under section 520(g) of the Federal Food, Drug, and Cosmetic Act means any activity that evaluates the safety or effectiveness of a device. [21 CFR 812.2(a)] Any activity in which results are being submitted to or held for inspection by FDA as part of an application for a research or marketing permit is considered to be FDA-regulated research. [21 CFR 50.3(c), 21 CFR 56.102(c)]. Human Subject as defined by the Common Rule: A living individual about whom an investigator (whether professional or student) conducting research obtains: 1. 2. data through intervention or interaction with the individual, or identifiable private information. Intervention includes both physical procedures by which data are gathered (for example, venipuncture) and manipulations of the subject or the subject's environment that are performed for research purposes. Interaction includes communication or interpersonal contact between investigator and subject. Private information includes information about behavior that occurs in a context in which an individual can reasonably expect Research, QI, Program Evaluation, EBP Tool Adapted with permission from COMIRB CF-195, Effective 9-20-13 COMIRB approved document that no observation or recording is taking place, and information which has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public (for example, a medical record). Private information must be individually identifiable (i.e., the identity of the subject is or may readily be ascertained by the investigator or associated with the information) in order for obtaining the information to constitute research involving human subjects.] Human Subject as Defined by FDA Regulations: Any individual who is or becomes a subject in research; either as a recipient of the test article or as a control. A subject may be either a healthy human or a patient. In the case of a medical device, a human subject/participant is also means a human on whose specimen an investigational device is used. Research Compared with Quality Improvement/Quality Assurance or Program Evaluation or EBP: The touchstones for separating quality improvement and program evaluation from research concern the intent of the project, the degree to which results are designed to contribute to generalized knowledge, the effect of results on program practice or processes, and the scope of dissemination of results. In general many research methods may also be used in quality improvement and program evaluation projects. In clinical settings the use of a placebo or significant deviation from standard of care is unlikely to be viewed as quality improvement or program evaluation. Quality Improvement: Quality Improvement Definition: Quality improvement activities are generally pursued in order to evaluate existing local practices with a goal of documenting and correcting deficiencies. If the goal of a project is to determine success/effectiveness or failure of a given program or process and the information gained from that evaluation is used to improve the program, this is not considered research involving human subjects, even when information is collected in a systematic way, because the results of this type of activity are not considered applicable to populations other than those under evaluation. Publication or presentation is allowed but results must not be described as or inferred to be generalizable to a broader population, i.e., they may not be described as research results. Quality Improvement Example: Research, QI, Program Evaluation, EBP Tool Adapted with permission from COMIRB CF-195, Effective 9-20-13 COMIRB approved document Routine lab audits revealed that the ED had a high blood culture contamination rate. The ED nurse manager shared this data with the staff and a clinical nurse volunteered to lead a Quality Improvement initiative to improve the contamination rate. Using a FOCUS-PDCA model, she identified sources of variation in practice, standardized a blood culture draw procedure, developed educational materials for the nurses and techs and competency verified all staff on the new procedure. Repeat audits demonstrated that the contamination rate decreased from 6.38% to 2.7%, which is below the national benchmark. Additional Guidelines for Quality Improvement: If, however, quality improvement activities involving human subjects are used to test novel services or programs for effectiveness and are presented in a more global fashion or applied to a broader population they should be considered research involving human subjects. For example: efforts to assess current clinic practices within a hospital (i.e., local) and to modify those practices to improve effectiveness would not meet the federal definition of research even though the evaluations collected data in a systematic manner. Presentation within the local environment (i.e., to the hospital staff) and publication of the results would be acceptable, so long as results are described as quality improvement and application of the findings is clearly limited to the location where they were found. If, however, results are presented outside of the local environment at a national meeting or published in a journal using language that seeks to generalize results beyond the locality of the project or that describes the study as research, the study would be considered human subject research and need review by the relevant IRB. Another example of research subject to IRB review would be efforts to assess current clinical practices of a number of local, unrelated entities and the aggregation of all these efforts to support a change in clinical practice beyond the local. As to each local organization, the assessment might constitute quality improvement, but when the results are aggregated to support a more generalizable recommendation, OHRP has determined that the aggregation of separate quality improvement activities constitutes research. Evidence-Based Practice Evidence-Based Practice Definition: Evidence-based practice is the synthesis and use of existing evidence to inform practice. Evidence Based Practice Example: Research, QI, Program Evaluation, EBP Tool Adapted with permission from COMIRB CF-195, Effective 9-20-13 COMIRB approved document Fever in patients with head injuries results in increased intracranial pressure and secondary brain injury. However, it was noted that management of fever in patients with traumatic brain injury varied widely in one neuroscience intensive care unit. A multidisciplinary team was formed who found, critiqued, and synthesized the literature. The resulting practice guideline delineated interventions for low, medium, and high fevers that progressed from noninvasive to invasive temperature management practices; a shivering guideline became an additional product. Evaluation of the guideline implementation resulted in a three-fold improvement in temperature management practices; monitoring of compliance with the guideline is ongoing. Program Evaluation: Program Evaluation Definition: The systematic collection of information about the activities, characteristics, and results of programs to make judgments about the program, improve or further develop program effectiveness, inform decisions about future programming, and/or increase understanding (Patton, 2008). Program evaluation is the inquiry into past, present, and potential programs to understand or clarify their needs, working processes, or impact. When the purpose of the evaluation is to provide feedback to the program and/or funder to improve that program, the activity is not human subject research and does not need IRB review and/or approval. Presentation of findings to the program and its funders and publication of the results would be acceptable, so long as results are described as program evaluation efforts and are clearly limited to the program to which they apply and are not described as research. Program evaluation is considered human subjects research when the intent is to contribute to generalizable knowledge. If results are presented or published using language that seeks to generalize results beyond the program studied, the study would be considered human subject research and would need review by the relevant IRB. Program Evaluation Examples: Examples of evaluations that would be considered research and need human subjects review include: Dissemination of evaluations connected to outcomes to affect the development or implementation of other programs similar in nature Evaluation undertaken to test a new, modified, or previously untested intervention, service, or program to determine whether it is effective and can be used elsewhere Research, QI, Program Evaluation, EBP Tool Adapted with permission from COMIRB CF-195, Effective 9-20-13 COMIRB approved document Other examples of program evaluation include: Evaluating the implementation of a state’s or county’s smoke free policies Evaluating a comprehensive cancer prevention and control project that uses media, health education, cancer screening promotion in a geographic region Evaluating the implementation of an evidence based practice in a clinical setting Evaluating an educational support program to transition associate degree nursing students to obtain a baccalaureate degree. Evaluating a diabetes management program conducted by a community clinic Even when activities constitute quality improvement or program evaluation, it is expected that the gathering of data from human subjects through direct or indirect interaction will be done with the highest level of regard for the protection of human subjects and in accord with ethical standards. The Project Lead (and mentor) should use this tool and the accompanying guidance to evaluate each project. Research, QI, Program Evaluation, EBP Tool Adapted with permission from COMIRB CF-195, Effective 9-20-13