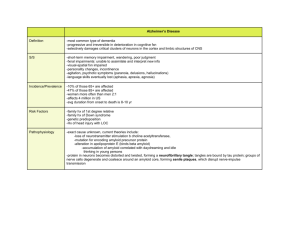
Alzheimer`s Disease: Formation of amyloid peptides and
advertisement

Alzheimer’s Disease: Formation of amyloid peptides and plaques in vivo Ben Zeskind and Stephanie Chang Alzheimer’s Disease (AD) is a neurodegenerative disorder affecting more than 4 million Americans, particularly those over the age of 65. Those afflicted with Alzheimer’s experience loss of short-term memory, dementia, and decreased mental capacity. Its causes are genetic, behavioral, and environmental. Over the past 15 years, researchers have begun to develop an understanding of the cellular and molecular mechanisms underlying the disease. Alzheimer’s initial causative event is the cleavage of a protein known as Amyloid Precursor Protein (APP) by - and -secretase instead of the normal -secretase. This produces a toxic A-42 peptide which forms insoluble extracellular aggregates. In combination with other proteins, A-42 forms plaques and long fibrils with cross-beta pleated sheet structure. When A-42 interacts with metals such as Fe2+ and Cu+, oxidative agents are created, which leads to damage of ion channels, cell surface receptors, and other vital cellular components. Damage to ion channels disrupts Ca2+ homeostasis, causing extensive cellular damage which includes hyperphosphorylation of tau, a microtubule protein. This altered form of tau contributes to the degradation of the cytoskeleton and to the formation of extracellular neurofibrillary tangles (NFTs). Image from Ingram Reference Below. Experiments over the past two decades have demonstrated that healthy cells produce soluble extracellular forms of A. Other research has established a correlation between genetic predisposition to AD and increased levels of extracellular A-42. Further studies have shown that elevated levels of dementia in patients are also connected with increased levels of extracellular A-42. Genetic predisposition to AD can be a result of mutations in the APP sequence which increase the likelihood that APP will be processed into A-42 instead of a more benign form. Mutations in other genes (such as PS1 and PS2) alter the activity of -secretase. Today’s therapeutic approaches to treating AD focus on several areas. Behavioral therapy includes low-fat, low cholesterol, seafood-rich diets (cholesterol also predisposes individuals to AD for reasons that are not fully understood but may involve altered membrane fluidity). Mental stimulation and an active lifestyle also reduce the risk of AD. More aggressive therapies currently under development involve using exogenous antibodies to remove A-42 from the brain, stimulating the body’s immune system to eliminate A-42, or blocking the action of -secretase. Less direct therapies include chelators, which reduce the action of Fe2+ and Cu+, preventing oxidative damage, and statins, which lower cholesterol. It is not yet clear that any of these therapies will cure Alzheimer’s, so significantly more research is needed. Selected References Esch, F.S., et al. “Cleavage of Amyloid Peptide During Constitutive Processing of Its Precursor.” Science. (1990) 248, 4959: 1122-1124. Haass, C., et al. “Amyloid- peptide is produced by cultured cells during normal metabolism.” Nature, (1992) 359, 322325. Haas, C. and Selkoe, D.J. “Cellular Processing of -Amyloid Precursor Protein and the Genesis of Amyloid -Peptide.” Cell. (1993) 75, 1039-1042. Ingram, V. “Alzheimer’s Disease,” American Scientist, (2003) 91, 312-321. Jackson Huang, T.H. “Structural Studies of Soluble Oligomers of the Alzheimer -Amyloid Peptide.” J. Mol. Biol. (2000) 297, 73-87 Mattson, Mark P. “Pathways towards and away from Alzheimer’s disease.” Nature, (5 Aug 2004) 203, 631-639. Naslund, J., et al. “Correlation Between Elevated Levels of Amyloid -Peptide in the Brain and Cognitive Decline.” JAMA, (2000) 283, 12: 1571-1577. Scheuner, D., et al. “Secreted amyloid -protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutationslinked to familial Alzheimer’s disease.” Nature Medicine, (1996) 2, 864-870. Selkoe, D.J. “Alzheimer’s Disease--Genotypes, Phenotype, and Treatments.” Science. (1997) 275, 5300: 630-631. Seubert, P., et al. “Isolation and quantification of soluble Alzheimer’s -peptide from biological fluids.” Nature. (1992) 359, 325-327. Wild-Bode, C., et al. “Intracellular generation and Accumulation of Amyloid -Peptide terminating at Amino Acid 42.” The Journal of Biological Chemistry, (1997) 272,26: 16085-16088.