This is a fictitious sample Statement of Work intended to assist
advertisement
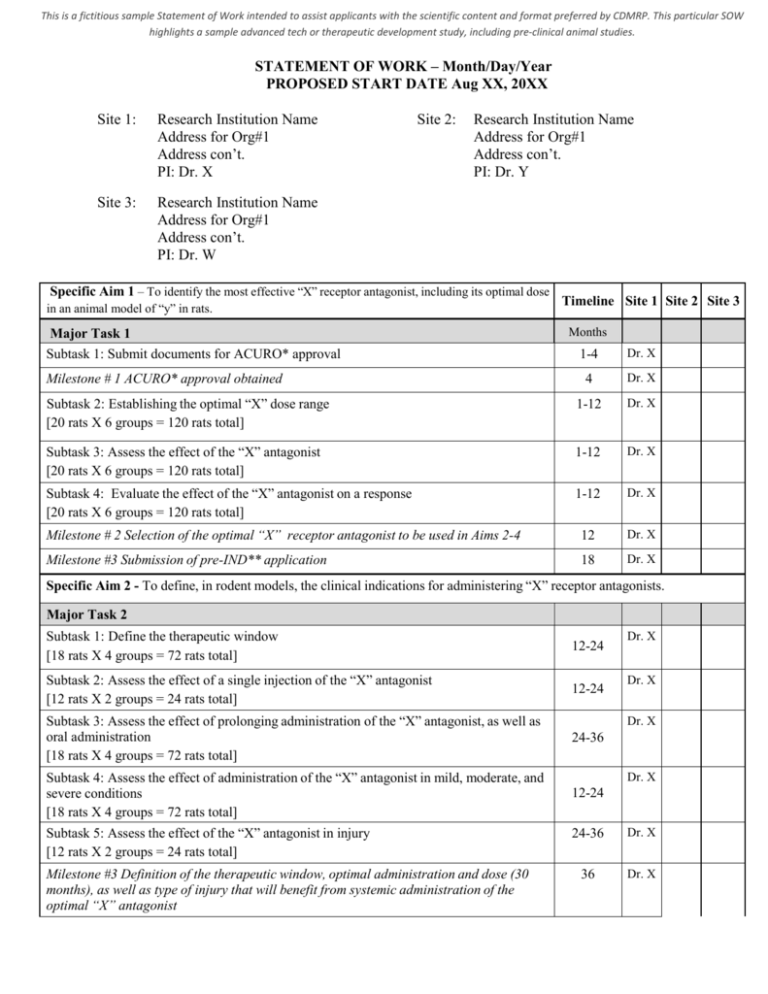
This is a fictitious sample Statement of Work intended to assist applicants with the scientific content and format preferred by CDMRP. This particular SOW highlights a sample advanced tech or therapeutic development study, including pre-clinical animal studies. STATEMENT OF WORK – Month/Day/Year PROPOSED START DATE Aug XX, 20XX Site 1: Research Institution Name Address for Org#1 Address con’t. PI: Dr. X Site 3: Research Institution Name Address for Org#1 Address con’t. PI: Dr. W Site 2: Research Institution Name Address for Org#1 Address con’t. PI: Dr. Y Specific Aim 1 – To identify the most effective “X” receptor antagonist, including its optimal dose Timeline Site 1 Site 2 Site 3 in an animal model of “y” in rats. Major Task 1 Subtask 1: Submit documents for ACURO* approval Months 1-4 Dr. X 4 Dr. X Subtask 2: Establishing the optimal “X” dose range [20 rats X 6 groups = 120 rats total] 1-12 Dr. X Subtask 3: Assess the effect of the “X” antagonist [20 rats X 6 groups = 120 rats total] 1-12 Dr. X Subtask 4: Evaluate the effect of the “X” antagonist on a response [20 rats X 6 groups = 120 rats total] 1-12 Dr. X Milestone # 2 Selection of the optimal “X” receptor antagonist to be used in Aims 2-4 12 Dr. X Milestone #3 Submission of pre-IND** application 18 Dr. X Milestone # 1 ACURO* approval obtained Specific Aim 2 - To define, in rodent models, the clinical indications for administering “X” receptor antagonists. Major Task 2 Subtask 1: Define the therapeutic window [18 rats X 4 groups = 72 rats total] 12-24 Subtask 2: Assess the effect of a single injection of the “X” antagonist [12 rats X 2 groups = 24 rats total] 12-24 Subtask 3: Assess the effect of prolonging administration of the “X” antagonist, as well as oral administration [18 rats X 4 groups = 72 rats total] 24-36 Subtask 4: Assess the effect of administration of the “X” antagonist in mild, moderate, and severe conditions [18 rats X 4 groups = 72 rats total] 12-24 Subtask 24 rats 5: Assess the effect of the “X” antagonist in injury [12 rats X 2 groups = 24 rats total] Milestone #3 Definition of the therapeutic window, optimal administration and dose (30 months), as well as type of injury that will benefit from systemic administration of the optimal “X” antagonist Dr. X Dr. X Dr. X Dr. X 24-36 Dr. X 36 Dr. X This is a fictitious sample Statement of Work intended to assist applicants with the scientific content and format preferred by CDMRP. This particular SOW highlights a sample advanced tech or therapeutic development study, including pre-clinical animal studies. Specific Aim 3 - To evaluate the pharmacokinetic, as well as idiosyncratic dose-dependent toxicity of the optimal “X” receptor antagonist defined in Aim 1. Major Task 3 Subtask 1: Establish the pharmacokinetics and bioavailability of the “X” receptor antagonist identified in Aim 1 as that with the optimal clinical profile. 12-24 Dr. Y Subtask 2: Establish the safety and toxicity profile of the optimally-effective “X” antagonist, following acute intravenous injection. 12-24 Dr. Y Milestone #4 Collect data needed for submission of IND application from outside contractor 24 Dr. Y Specific Aim 4 – To identify all spinal cord cell types expressing “X” after as well as before injury, and to define the effects of injury on “X”-regulated gene expression in those cells. Major Task 4 Subtask 1: Defining cell types expressing “X” before and after injury in animal model [25 mice X 2 groups = 50 mice total] 1-12 Dr. W Subtask 2: Assess the transcriptional effects of injury on “X” expressing cells in animal model [12 mice X 2 groups = 24 mice total] 12-24 Dr. W Subtask 3: Assess the transcriptional effects of “X” inhibition after injury in “X”+cells [12 mice X 2 groups = 24 mice total] 24-36 Dr.W Milestone #5 Definition of cellular targets for “X” receptor inhibition. Defining cell specific signaling pathways, activated by injury and their dependence on “X” receptor. 30-36 Milestone #6 Submission of full IND** application 30 Dr. X * ACURO = Animal Care and Use Review Office; review and approval by ACURO office of animal protocols is required of all DoDfunded awards including animal research ** IND = Investigational New Drug; FDA requirement for new investigational drugs prior to their use in human research clinical trials





