Ch 6: Carbonyl Complexes and Hydrido Complexes
advertisement
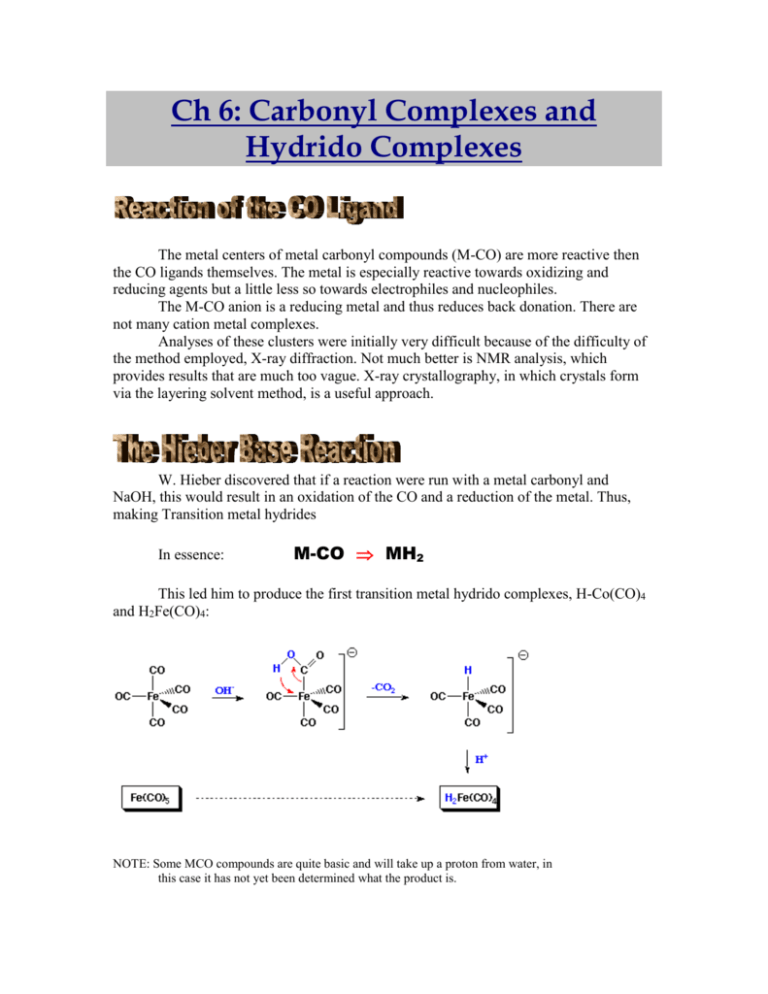
Ch 6: Carbonyl Complexes and Hydrido Complexes The metal centers of metal carbonyl compounds (M-CO) are more reactive then the CO ligands themselves. The metal is especially reactive towards oxidizing and reducing agents but a little less so towards electrophiles and nucleophiles. The M-CO anion is a reducing metal and thus reduces back donation. There are not many cation metal complexes. Analyses of these clusters were initially very difficult because of the difficulty of the method employed, X-ray diffraction. Not much better is NMR analysis, which provides results that are much too vague. X-ray crystallography, in which crystals form via the layering solvent method, is a useful approach. W. Hieber discovered that if a reaction were run with a metal carbonyl and NaOH, this would result in an oxidation of the CO and a reduction of the metal. Thus, making Transition metal hydrides In essence: M-CO MH2 This led him to produce the first transition metal hydrido complexes, H-Co(CO)4 and H2Fe(CO)4: NOTE: Some MCO compounds are quite basic and will take up a proton from water, in this case it has not yet been determined what the product is. How acidic or how basic are these transition metal hydrido compounds? Complex IR (M-H) 1H-NMR pKa mp (C) Dec. 1 2 3 HCo(CO)4 H2Fe(CO)4 HMn(CO)5 1 pKa = 4.7, 2 ST ND -26 -70 -25 -25 -10 >30 1934 1783 -10 ppm -11 ppm -7.5 ppm 1 4.7 7 Comparable acid H2SO4 AcOH H2S pKa = 14 H2O It is important to note that –10 and –11 ppm are outside the usual range for the 1H-NMR spectrum, thus, when running the test the range must be increased. Also note, that with NMR spectrums, TMS is a good standard reference because nothing is to the right of it. With compound #1, as with many of the compounds, it is evident by the pKa that they can be very acidic. A second method of obtaining carbonyl hydride complexes did so via a formylcomplex intermediate. This method involves a reaction between an M-CO with a hydride donor such that a decarboxylation occurs: Formyl-complexes are extremely unstable and were never isolated however, in 1975 C. Casey characterized the complexes. Metal hydrido complexes are important in terms of their role in catalytic processes. For example, in hydroformylation reactions, a metal-hydrido catalyst HCo(CO)4, is used. (This catalyst is formed in situ from Co2(CO)8 and H2). Reactions in which metal-metal bonds are cleaved are usually thermodynamically favored. However, high temperatures and pressures are required:] (A) M—M + H2 M—H + M—H More indirect methods can be applied: (B) M—M + I2 M—I + M—I + H- M—H + M—H And (C ) M—M + 2e- M- + M- + H+ M—H + M—H Note: reduction of the halo carbonyl complexes are usually performed with NaBH 4, LiBH4, or BH3 Not all TM hydrido compounds are unstable, K2ReH9 is stable even at ~200C. The compound is made from the reduction of potassium perrhenate with metallic potassium in wet ethylenediamine. The product was first published as K[Re(H2O)4]. From this one can conclude the Golden Rule of Chemistry: One Method is No Method It took five methods to deduce the correct structure Try 1 2 3 4 5 Problem - the reduction worked and it was concluded and published that oxidation state was +1. - This was incorrect as Re is a heavy metal and below the favored coordination number 5, is very rare. - It was a wrong publication - IR method used, found coordination number of Re to be 8, this was wrong as well - elemental analysis produced wrong results as well - x-ray diffraction, produced a bad structure, the crystallographer was experienced only in this and therefore believed this was the structure - Neutron diffraction, found the correct structure In 1976, Ashworth and Singleton postulated the complexation of dihydrogen at transition metal centers. The bonding is as such The H—H have 2e- that are donated to the M empty orbital, the orbital of the ligand. Nature’s way of dihydrogen compounds are in enzymes. The first paper was published in 1984, in which Kubas synthesized a series of Mo(o)-complexes that reversibly add H2. Found that the compound obtained 18VE by picking the H2 up. Evidence for the 2-coordination of H2 came from single crystal Xray crystallography of the complex (Cy3P)2W(CO)3(H-D). With NMR, if the molecule is symmetric, it will not pick up coupling. This is the case with H-H, however, a complex with H-D will produce coupling ~33.5Hz. An IR band at 2360 cm-1 also indicates the H-D bond. (Particularly since there are not many peaks in this region to confuse the cause of this one) Dihydrogen complexes require both -donation and -back donation thus electron rich metal centers provide this electron density. The dihydrogen complexes can be acidic with pKa values of 0-10. Most complexes have the transition metal in the d6 configuration. It is still possible to add to the square planar metals in configuration d8 but these reactions lead to classical complexes. These reactions are the elementary step of homogeneously catalyzed hydrogenations. The H2 molecule seems to add in a way that eliminated trans isomers. For the Vaska complex, the addition of H2 can be along the Cl axis or the PPh3 axis. However, only one isomer is observed. The one resulting from addition of the Cl-Ir-CO axis. (These results are not normally the case) Tremendous diversity of these complexes arises from the stability of the M-M bonds (particularly Fe, Co and Ni) and the compatibility with many other CO-ligands. For example, we can have mono-, di-, tri-, and polynuclear complexes (obtained by heating MCO’s until CO evolution starts). With Triiron-dodecacarbonyl, it was estimated that the compound was symmetric but it is not. One Fe has 4 terminal ligands Another Fe has only 3 terminal ligands but there is a bridging CO ligand, which shortens the Fe-Fe bond distance (I): X-Ray Crystallography The M-C and O-C bond distances in carbonyl complexes do not vary much but can be determined by single X-ray crystallography. The M-C-O fragment is rarely linear, mostly bent. X-ray crystallography provides information about the number of ligands around the metal and the presence of unusual coordination modes (II) 13 C-NMR-Spectroscopy MCO-s are typically reactive, especially if UV light is shone on them. The light causes a charge transfer, the transport of e—density from M to the CO ligand, which goes into the anti-bonding levels. h 2 Re(CO)5Br CCL4 -2CO Br (CO)4Re (CO)4Re Br Here lone pairs on Bromine allows it to dimerize forming a strong lewis acid CO 2C5H5Co(CO)2 h -2CO (C5H5)Co==============Co(C5H5) CO Here the M=M double bond allows the two to share electrons and gain a valence electron count of 18. Reacting Fe(CO)5 with Na, which forms Na2Fe(CO)4, can obtain this compound. This compound, which is the dianion of Fe(CO)4, is not very stable but can be stabilized as a complex with dioxane. The procedure allows you to replace an organic halide with a formyl group in an extremely selective manner. Two types of reactions can proceed: In this reaction, CO inserts itself into the C-Halogen bond and is then hydrogenated with an acid. As can be seen, Br is more reactive then Cl, however both are much better then I. Another reaction can be performed to produce a ketone. Instead of protonating with an acid, use another organic compound This reaction is a multi component, 3, condensation and produces a ring. It is one of the only good multi-component reactions because this can make many useful compounds. For example, a family run business in Switzerland, Butaco, makes fragrances through this three-component reaction. The reaction takes CO + alkene + Alkyne and produces cyclopentanones. Chapter 7: Alkyl Complexes, Carbene Complexes, and Carbyne Complexes Organic compounds of the transition elements differ from those of the main group elements for many reasons, most obvious is the difference in thermal stability. The low thermal stability of transition metal alkyl complexes is due to the existence of a number of low energy decomposition pathways that are specific to transition metals. For example The instability of this compound can be accounted for by its highly electron deficient character (8 VE instead of 18). Decomposing at -70C. How can these compounds be stabilized? Since they decompose through a certain mechanism. In this case the reaction proceeds through a -elimination. M C H C M-H + ethylene. Thus, a good method would be the blockage of this mechanism by making sure the alkyl C does not have a -hydrogen . Wilkinson set out to do this by building compounds with more bulk that are higher in stability. the substituents, CH2SiMe2, increase the VE count (to 14 VE) and therefore stabilize the Ti-Me bond. This compound sublimes at 90C. You can tell if there is a -hydrogen because of the characteristics of this type of interaction, Agostic Interaction. This was discovered by M. L. H.Green, and is common with early transition metals, or electron deficient metals; Ti, V, Zn, Nb etc. Has unusual 1H shifts because M C H C Of strange shielding Also has low (C-H) which is ~3000cm-1 usually but drops in this case ~2600-2900cm-1. Other effective stabilizers are covalent ligands with lone pairs. Stability is not restricted to singly bound ligands, doubly bound oxygen and nitrogen and triply bound nitrogen also greatly increase the stability of the compounds. Monomolecular pathways do not require an encounter of two different molecules, like bimolecular pathways. As such, they are difficult to block by the introduction of bulky auxiliary ligands As demonstrated by J. Okuda, it is likely that bimolecular pathways are important, particularly for complexes of type CpTiMe3. UNSTABLE Mp = +58C, dec > 120C Sublimes w/o decomposition A number of factors are involved with stability. These are Stability of Alkyl Complexes 5d > 4d > > 3d late TM > early TM M in high CN > M in low CN 18 VE > 16 VE > 17, 19 VE cyclic > open chain Os , Re, Pt > others Combining these rules increases the stability of complexes. For example, G. Whitesides investigated the stable cyclic platinum complex formed in this reaction: In this reaction alkynes react with the Schwartz Reagent Cp2Zr(Cl)H, and produces vinyl zirconium, these compounds favor 1,4-addition to Michael systems. R Cp2Zr(Cl)H Cl Cp2Zr R H Note: Under certain conditions the Zr can migrate. You can basically use these reagents as vinyl anions “vinyl -” Overall, REAGENT M--Cl RMgX R – Li R2Zr R3Al…R2AlCl (R3B) Can also perform hydrometalations: M—H OR M-C-C-H M—H C: M-C-H Or Decarbonylations: M(CH3)C=O -CO M-CH3 Or Decarboxylations: Hg2+ (CF3COO-)2 / -CO2 (CF3)2Hg Note: this is limited to Cd, Bi, (As), (Sb), Pt and Au The first compounds with a double bond between a transition metal and a carbon, in the Carbene Complex, first isolated by E. O. Fischer and Maasbol in 1964: Note, that the product has the same valence electron count because the substituent :C(OCH3)2, donates 2 e- just like the CO does. A number of interdependent characterizations of the general Fischer Carbene Complexes are: L: CO, isonitril, Cp, PR3 and other ligands that act as –M groups M: Cr > Mn > Fe triads with oxidation states < 2 R: At least one donor group is required (-OR, SR, NR2) Reactvity: Dominated by electrophilic M=C carbon Note: that reactivity has to do with the mesomeric structure associated with the double bond, which inhibits rotation: M+—C-—Do R CO—M=C—Do R A new carbene complex was discovered by R. Schrock in 1974 in an attempt to obtain pentakis(neopentyl)tantalum(V), which was discovered to be the intermediate. This formed an “Alkylidene”, where the substituent is bound by a double bond: By: Marsha Youash 970168220 Prof. Denk March 16, 2000






