Exempt - Clemson University
advertisement

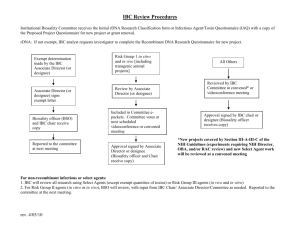
IBC Protocol Instructions Approved 5/13/02 CLEMSON UNIVERSITY RESEARCH and TEACHING INVOLVING HAZARDOUS AGENTS INSTRUCTIONS, CHECKLISTS and FORMS FOR COMPLETION OVERVIEW: Clemson University aims to provide a safe and healthful work environment for employees, students and visitors. It is the intent to minimize to the extent practicable all recognizable hazards and to comply with all federal and State laws and regulations. In accordance with federal regulations, the Institutional Biosafety Committee (IBC) was established to provide review of all protocols that involve the use of hazardous or potentially hazardous agents. By providing the following information, you assist our committee in meeting the standards set by the federal government and Clemson University. The Chief Environmental Health and Safety Officer (CEHSO) serves as the University Biosafety Officer (BSO) and must be consulted regarding any proposed use of potentially hazardous agents. EHS is responsible for the maintenance of a biosafe environment at Clemson, and they work with the principal investigator (PI) who is directly responsible for adherence to biosafety regulations in his/her laboratory. This includes the actions of all students and technicians. Upon request, Chief Environmental Health and Safety Officer (656-1806) will assist investigators in developing protocols. REVIEW PROCESS: To expedite the review process the IBC utilizes three levels of review based on the nature of the hazard(s) involved; Exempt, Designated and Full Committee. EXEMPT PROTOCOLS are those that present only minor hazards with very low risk that may be addressed in a short form that is reviewed by the BSO and IBC chair to validate the Exempt status. Exempt protocols may be validated immediately upon review by the BSO and IBC chair and require continuing review on an annual basis. DESIGNATED REVIEW is used for protocols that present a moderate risk and require review by the BSO, IBC chair and usually at least one other IBC member with the appropriate expertise (designated by the IBC chair). Protocols involving chemicals that are highly toxic, carcinogenic, mutagenic or teratogenic may be reviewed as designated at the discretion of the chair and BSO. Recombinant DNA Protocols assigned designated review must be reviewed by five IBC members in compliance with federal guidelines for the use of recombinant DNA. Designated Review requires 5 to 10 working days and approval may be granted once concerns, if any, have been adequately addressed by the PI. These protocols require continuing review on at least an annual basis. FULL COMMITTEE REVIEW is used for protocols that may present significant hazards to humans, animals or the environment. Examples of significant hazards would be work with biological agents requiring greater than BSL2 containment facilities or practices or release of genetically altered organisms. All members of the IBC participate in a Full Committee Review, which may require up to 10 - 15 working days depending on the complexity of the proposed work. In some cases, the IBC chair may request a meeting of the IBC to discuss concerns related to a full committee review. Protocols requiring Full Committee Review may be approved as soon as all concerns have been adequately addressed by the PI. These protocols require continuing review on at least an annual basis and more frequently if indicated. IBC Protocol Instructions Approved 5/13/02 Any proposed changes in the protocol for the use of hazardous agents must be submitted and approved as an update prior to initiation. An annual review of the hazardous agent use must be completed and approved at least every twelve (12) months for the study to continue. INSTRUCTIONS: The following Protocol should be completed and signed by the Principal Investigator and Department Chair. Please submit original signature page and hazard acknowledgement signature sheet to the Office of Research Compliance, E308 Martin Hall. This form will not be considered complete until all of the items have been addressed (requested information that is not available should be noted). Please note specific instructions for each type of biohazard (biological, chemical, and recombinant DNA). Incomplete forms will not be approved. Should you have any questions, please do not hesitate to contact the Office of Research Compliance at 656-6460. ANIMALS: REQUIRED INFORMATION: Complete an Animal Use Protocol and submit it to the ARC Complete the relevant parts of the Hazardous Agent Protocol Include an abstract and flow sheet of the proposed research; and BIOLOGICAL HAZARDS: A biohazard is a potentially dangerous infectious agent or material (tissue, blood, etc.) that is suspected to contain an infectious agent or whose hazard status is unknown. For our purposes, a biohazard is any BSL2 agent or above, or human or animal blood, tissue, or waste specimen known to harbor infectious agents or whose biohazard status is unknown; human or non-human primate derived cell lines or similar are also considered biohazards (per OSHA definition). Infectious organisms includes all agents (including prions) capable of causing disease in healthy humans or animals, whether these occur commonly in the environment or not. The Center for Disease Control-NIH Biosafety in Microbiological and Biomedical Laboratories http://www.cdc.gov/od/ohs/biosfty/bmbl4/bmbl4toc.htm manual should be used for guidelines in completing the Biohazards Protocol section. It is a requirement that all personnel be offered appropriate inoculations against any pathogenic for any occupational health purposes before any lab work can be done. If inoculations are not received, a waiver must be signed. Regardless of the source, if using human or non-human primate tissue or body fluid(s), a protocol will be required. REQUIRED INFORMATION: If the Protocol will Qualify as Exempt: Completed Signature Page The Exempt Certification Form General Information: Protocol Qualifying as Exempt; and All individuals working on the project must sign the Hazard Acknowledgement Signature Sheet (or a document in your own wording, if necessary) indicating they are aware of the potential hazards of the protocol. If the Protocol Does Not Qualify as Exempt: Completed Signature Page General Information: Protocol Not Qualifying as Exempt Part B; and IBC Protocol Instructions Approved 5/13/02 All individuals working on the project must sign the Hazard Acknowledgement Signature Sheet (or a document in your own wording, if necessary) indicating they are aware of the potential hazards of the protocol. CHEMICAL HAZARDS: Any chemical listed as highly toxic, carcinogenic (confirmed or suspected), mutagenic, teratogenic or explosive on its MSDS must be covered by an approved protocol; if undergraduates are involved in the research, any chemical listed as toxic, carcinogenic (confirmed or suspected), teratogenic or explosive on its MSDS must be covered and the protocol will not qualify as Exempt. Highly toxic is defined as: 1) having an oral LD50 of 50 mg/kg body weight when administered orally to albino rats weighing between 200 and 300 g each. 2) LD50 (200 mg/kg body weight when administered by continuous contact for 24 hours (or less if death occurs within 24 hours) with bare skin of albino rabbits weighing between 2 and 3 kg each. 3) LC50 in air of 200 ppm by volume or less of gas or vapor, or 2 mg/l or less of mist, fume or dust, when administered by continuous inhalation for one hour (or less if death occurs within one hour) to albino rats weighing between 200 and 300 g each. Toxic is defined as: 1) having an oral LD50 (500 mg/kg 2) a contact LD50 of (1000 mg/kg for 24 hour exposure or 3) LC50 of 2000 ppm with one hour exposure. If this information is not listed for rats (or rabbits for contact), and any test animal listed has an LD50 lower than the amounts listed above; the chemical must be covered by an approved protocol. Also, chemicals having an LD50 for humans listed at amounts below those listed above must be covered by an approved protocol. REQUIRED INFORMATION: If the Protocol will Qualify as Exempt: Completed Signature Page The Exempt Certification Form General Information: Protocol Qualifying as Exempt Manufacturer’s Material Safety Data Sheets (MSDS) for each chemical listed (only attach paper copy if not submitting electronically or the chemical is not listed in the CCINFO MSDS database (accessible from the EHS website). It allows you to search and print chemical data from six databases; and All individuals working on the project must sign the Hazard Acknowledgement Signature Sheet (or a document in your own wording, if necessary) indicating they are aware of the potential hazards of the protocol. If the Protocol Does Not Qualify as Exempt: Completed Signature Page General Information: Protocol Not Qualifying as Exempt Part C Manufacturer’s Material Safety Data Sheets (MSDS) for each chemical listed (only attach paper copy if not submitting electronically or the chemical is not listed in the CCINFO MSDS database (accessible from the EHS website). It allows you to search and print chemical data from six databases; and All individuals working on the project must sign the Hazard Acknowledgement Signature Sheet (or a document in your own IBC Protocol Instructions Approved 5/13/02 wording, if necessary) indicating they are aware of the potential hazards of the protocol. See the following websites for copies of MSDS: http://ehs.clemson.edu/ RECOMBINANT DNA: By definition (Federal Register 51 (88) page 16959 I-D-2), the CU IBC reviews and oversees projects which deal with recombinant DNA (rDNA) technologies. Clemson University must have such a committee made up of faculty, staff, and community members, for review of protocols and compliance in rDNA matters. While the most scrutinized protocols are those dealing with the environmental release of genetically engineered organisms, all protocols including those using only laboratory contained experiments are closely examined. CU has a policy of requesting that all investigators file a protocol when using rDNA molecules or organisms, although certain types of experiments will qualify as "Exempt". This process guarantees our compliance with Federal regulations and allows us to assure the public that we are safeguarding the public interest. If you work with recombinant DNA, (see Section I-B of the NIH Guidelines: Definition of Recombinant DNA Molecules), a component of the protocol form will require you to identify the section(s) and appendices of the NIH Guidelines appropriate for your experiments. A copy of the Guidelines for Research Involving Recombinant DNA Molecules is available on the Internet at http://www.nih.gov/od/oba/. A hard copy is available in the Office of Research Compliance. If you plan at any time to introduce genetically engineered organisms into the environment, additional information must be filed. For this component, you will need to complete Steps 1-4 in Part D of the protocol. You also will need to reference the USDA publication entitled Guidelines for Research Involving Planned Introduction into the Environment of Genetically Modified Organisms (December 3-4, 1991 or most current version). REQUIRED INFORMATION: If the Protocol will Qualify as Exempt: Completed Signature Page The Exempt Certification Form General Information: Protocol Qualifying as Exempt; and All individuals working on the project must sign the Hazard Acknowledgement Signature Sheet (or a document in your own wording, if necessary) indicating they are aware of the potential hazards of the protocol. If the Protocol Does Not Qualify as Exempt: Completed Signature Page General Information: Protocol Not Qualifying as Exempt Part D; and All individuals working on the project must sign the Hazard Acknowledgement Signature Sheet (or a document in your own wording, if necessary) indicating they are aware of the potential hazards of the protocol. IBC Protocol Exempt Certification Form INSTITUTIONAL BIOSAFETY COMMITTEE: Approved 5/13/02 EXEMPT CERTIFICATION FORM Protocols that present only minor hazards with very low risk to humans, animals or the environment may be exempt from full review of the IBC. Exempt protocols may be validated immediately upon review of the CHESO and IBC chair and require continuing review on an annual basis. Please complete the following checklist to determine if your study may be validated as exempt. Use of any non-exempt agent requires the submission of a non-exempt protocol application for all agents. Hazard Categories Check List (Check all that apply): Animal Use: This proposal involves the use of live animals. An Animal Use Protocol must be submitted to the ARC, and any research or teaching involving a hazardous agent will require approval by the IBC. Biological Hazard [Refer to Center for Disease Control-NIH Biosafety in Microbiological and Biomedical Laboratories (Current Edition) Does this protocol involve biological agents that require containment levels of: Biosafety Level 4 (BSL4)/(ABSL4) YES NO Biosafety Level 3 (BSL3))/(ABSL3) YES NO Biosafety Level 2 (BSL2))/(ABSL2) YES NO Biosafety Level 1 (BSL1))/(ABSL1) YES NO CDC select agents YES NO Complete Section B if this proposal requires levels 2, 3 or 4. Protocols requiring containment levels of BSL2 or above and/or undergraduate students will not qualify for exempt. Chemical Hazard (Consult Material Safety Data Sheets (MSDS) for this information) This proposal does involve chemicals that are: highly toxic mutagenic teratogenic carcinogenic (confirmed or suspected) explosive YES YES YES YES YES NO NO NO NO NO Complete Section C if this proposal does involve any of the above categories. Protocols involving any of the above categories will not qualify as exempt. Protocols involving undergraduate students will not qualify for exempt. Recombinant DNA (Refer to NIH Guidelines for Research Involving Recombinant DNA Molecules for more information) This protocol does involve: the planned release of an experimental genetically modified/engineered (transgenic) organism into the environment (excluding contained areas such as greenhouse, growth chamber or laboratory) the direct transfer of a gene or DNA derived from any source (or RNA derived from recombinant DNA) into a human genes encoding animal/human toxins (or their toxic subunits) (e.g., those on the CDC’s Select Agent List or listed in Appendix A of the NIH Guidelines) the cloning of a gene or factor associated with an animal/human disease recombinant molecules that contain the majority of a pathogen's genome (e.g., those on the CDC’s Select Agent List, or in the NIH’s Risk Group 2, 3 or 4) the use of organisms from the CDC’s Select Agent List, or in NIH Risk Groups 2, 3 or 4, as hosts for the propagation or expression of recombinant DNA molecules Complete Section D if this proposal does involve any of the above categories. If you checked any of the above categories, your proposal does not qualify as exempt. CLEMSON UNIVERSITY HAZARDOUS AGENTS PROTOCOL - EXEMPT SIGNATURE PAGE Protocol #:_________________________ AUP or IRB #:__________________ Date Approved: _____________________ Signature IBC Chair: _______________________________________________________ Signature CEHSO: ____________________________________________________________ (For Office of Research Compliance use Only) This is a: New Protocol Revised Protocol Amendment Hazard Category: (check all that apply): Biological Chemical rDNA Animals will be involved in this protocol: Human subjects or tissue(s) will be involved in this protocol: Yes Yes No No If you answered Yes to either of these questions, please submit the appropriate IRB or ARC Protocol Application. ARC or IRB Protocol Number if known: ) Principal Investigator: Co-Investigator: Full Name of Principal Investigator Full Name of Co-Investigator E-Mail Address Department Telephone Number Fax Number Mailing Address Sponsor Information Sponsored Programs Proposal # Project or CourseTitle: (must match the title submitted to the funding agency, departmental course coordinator or any other CU committee) Source of Funding: Anticipated Start Date of Project: Expected Duration of Project (indicate if months, years, etc.): Signature of Principal Investigator: _________________________ Signature of Co-Investigator: ______________________________ Date: _________ Date: _________ Signature of the principal investigator certifies that the information in this protocol is accurate and complete, that the PI is familiar with federal and institutional regulations and guidelines and agrees to abide by such regulations and guidelines. Signature also certifies that the PI is responsible for assuring that all personnel working with the project are properly trained and informed of the hazards involved and have signed a statement indicating that they have been informed of such hazards. Signature of Department Chair: ____________________________ Date: __________ Signature of the department chair certifies that he/she is familiar with the project, is aware of the hazards involved and that the project has been given his/her approval Part A- General Information Approved 5/13/02 PART A - GENERAL INFORMATION HAZARDOUS AGENTS PROTOCOL FORM To be filled out for: PROTOCOLS QUALIFYING AS EXEMPT 1. AGENTS UTILIZED A. Names of Agent(s): Biological Chemical rDNA B. 2. Provide a short synopsis in non-technical language of how the agent (chemical, biologic and/or rDNA) is to be used. An abstract or means and methods may be attached. This is to help IBC members and non-specialists understand the nature of the project and its significance. HANDLING OF AGENT A. Personnel Conducting Research (faculty, staff and students): Name B. Title/Status Training & Experience in Research Designated Work Areas: (building, room, labs, storage areas, etc.) Building Room Number Type of Room Part A- General Information 3. Approved 5/13/02 HAZARDOUS AGENT IDENTIFICATION A. (1) What hazardous agent signage is being used? Biohazard Chemical Other (2) Are the hazardous agents identified on these signs? Yes No (3) Where is hazardous agent signage being used? Lab entrance Storage areas (refrigerators, freezers, etc.) Work areas (biosafety cabinet, incubators, etc.) Other: (please specify) (4) Contact numbers for emergencies have been posted on the laboratory doors. Yes No B. 4. The signs are incompliance with the requirements of "Biosafety in Microbiological and Biomedical Laboratories" and/or appropriate to your Chemical Hygiene Plan. Yes No (i.e for BSL-2/ABSL-2 or above: a biohazard sign MUST be posted at the entrance of the laboratory when etiologic agents are in use. The sign will include the name of the agents in use, the biosafety level and the name and phone number of the Investigator and any personal protective equipment which must be worn in the laboratory and any procedures required for exiting the laboratory) GENERAL LABORATORY INFORMATION A. Is there a current Clemson University Chemical Hygiene Plan available for all laboratory and storage facilities? YES, Plan is dated Copies are maintained in (bldg./room). YES, Individual laboratory Standard Operating Procedures are available. Copies are maintained in (bldg./room). NO (Contact EHS for information) Biological Safety Officer: Mr. Robin Newberry 656-1806 or wnewber@clemson.edu Chemical Safety: Ms. Naomi Kelly 656-7554 or nkelly@clemson.edu Part A- General Information Approved 5/13/02 B. Laboratory Facilities and Procedures are in Compliance with standard microbiological practices as described in the current edition of "Biosafety in Microbiological and Biomedical Laboratories" Yes No C. Safety Equipment and Protective Apparel (Must be consistent with MSD Sheets and other guidelines): Describe protective equipment (fume hoods, biosafety cabinets, etc.) and apparel (face shield, goggles, dust/mist mask, NIOSH approved respirator, etc.). If equipment is such that inspection or training is required, include date of inspection or training. D. Organisms Genetically Modified through Recombinant DNA Methodologies 1. Are transgenic organisms, other than commercially available strains, involved? YES 2. NO (no further information necessary in this section) Will the genetically modified micro-organism(s) be grown in quantities greater than ten (10) liters: (see Appendix K in the NIH Guidelines for Research Involving Recombinant DNA Molecules) YES NO If Yes, what volume: _____________ 3. Will the genetically modified organism(s) be: … released into the environment? YES NO If yes, be sure to complete all of Section D … maintained in the laboratory or other controlled environment ? YES NO Explain procedures used to avoid GMO/agent escape (containment). Hazard Acknowledgement Sheet Approved 5/13/02 HAZARD ACKNOWLEDGEMENT SIGNATURE SHEET Protocol #: _________________ Cross-Reference #:____________ Principal Investigator: _________________________________________ Project Title: _________________________________________________ ____________________________________________________________ I have read, understand, and agree to abide by the Hazardous Agents Protocol listed above when working with the hazardous agents described therein. I understand the nature of the hazard associated with the agents under this protocol. I understand that disregard for the policies and procedures as outlined in this protocol could result in dismissal from work with the designated hazardous agents. Date Name (Print) Signature __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________ __________________________________________ __________________