Drugs_that_Affect_Coagulation_Lecture_Objectives
advertisement

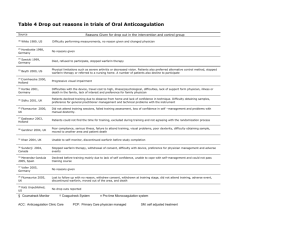
Drugs that Affect Coagulation: Lecture Objectives. Fitzakerley. 05. 07. 09. Katelyn Rogers 1. Be able to diagram the coagulation & fibrinolytic pathways, including the interaction of protein C w/ those pathways (Sketch on separate sheet). Define how pro- & anti-coagulant drugs (including thrombolytics & antiplatelet drugs) interact w/ specific clotting factors & naturally occurring molecules in these pathways. (On sketch label where the drugs interact w/ the factors) 2. Be able to identify both the common & the distinguishing characteristics of thrombolytic agents. Describe the similarities & differences btwn streptokinase/urokinase & tPAs. Common characteristics of thrombolytic agents: Dissolve clots Process of changing/moving plasmin so it can be active Given IV Short activation & t ½ Used for recent MI (6hrs), PE, & DVT Distinguishing characteristics of thrombolytic agents: STREPTOKINASE: If patient is allergic to STREPTOKINASE use UROKINASE a human kidney enzyme that converts plasminogenplasmin, which has only fever & no anaphylaxis for adverse effects. Common contraindications/Precautions of thrombolytic agents: Cause bleeding/hemorrhage Therefore, don’t administer post-surgery, post-trauma, hemorrhagic strokes, pregnancy, or GI bleed/ulcer Contraindicated w/ hypertension Mech of action: not intrinsically active, causes a conformational Δ in plasminogen exposing active site. Adverse effects: isolated from bacteria, allergic rxn includes anaphylaxis & fever. tPAs (human melanoma cells): ALTEPLASE (unmodfd), RETEPLASE (deleted some aas), TENECTEPLASE (substituted): Mech of action: selective activation of fibrin bound plasminogen (easy target). Only fibrinolytics approved for use in non-hemorrhagic stroke (3hrs). 3. Compare & contrast: Green means good things. With respect to mechanism of action, pharmacokinetics (esp. time to onset of activity), method of monitoring, antidotes, use during pregnancy & side effects. a. Heparin & low molecular weight heparins HEPARIN LMWHs: DALTEPARIN & ENOXAPARIN Size (MW) Big Small Anti-thrombin (Anti-IIa) High Low Anti-Xa Low High Bioavailability Low High T1/2 (leading to ↓ dosing) Shorter Longer Predictability Poor Good Platelet inhibition More Less Plasma protein bound More Less-not displaced as easily HIT More Less Monitor All the time Only during pregnancy Antidote (PROTAMINE High Low SULFATE) efficiency b. Heparin & direct thrombin inhibitors (DTIs) & Warfarin. HEPARIN Mech of action Pharmacokinetics *time of onset Requires AT III binding (IIa,VIIa,IXa, Xa, XIa, XIIa) & coats bv wall ↓ platelet binding & ↑ permeabilization. IV or SC *RAPID onset Liver metabolism Kidney excretion DTIs: ARGATROBAN, BIVALIRUDIN, HIRUDIN, LEPIRUDIN Interact directly w/ thrombin (IIa) RUDINs- irreversible ARGATROBAN- comp antagonist WARFARIN IV RUDINs- kidney excretion ARGATROBAN- liver metabolism Oral *RAPID onset Narrow TI 99% bound to plasma proteins (easily displaced) S form (CYP2C) active- other drugs affect it R form (CYP1A2, 2C19, 3A4)- affects other drugs Gene modifications can affect metabolism Slow CYP2C9- ↑ serum conc Resistant VKOR-↓ effectiveness Coag cascade also resistance (Fact V Leid)- -↓ effectiveness No in vitro effect INR- correlates w/ factor II. Protein C & Factor VII deplete first, causes coagulation temporarily. Last is factor II (prothrombin). PHYTONADIONE (Vitamin K) Monitoring method Frequent aPTT Sometimes aPTT ARGATROBAN- ↑es INR, therefore can’t be used. Antidotes PROTAMINE SULFATE NONE- irreversible, except ARGATROBAN is reversible, but no agents exist Use during pregnancy Yes- doesn’t cross placenta Bleeding, hypersensitivity which can progress to anaphylaxis, HIT. Prolonged exposure: osteoporosis, ↓ed AT III leading to ↑ed clotting, & a mineracorticoid defiency bc of ↓ aldosterone. HEPARIN for immed DVT, PE, MI, etc In combination w/ thrombolytics & GPIIb/IIIa inhibitors for angioplasty or stent placement. Side effects Therapeutic Uses Bleeding, anaphylaxis, Abproduction against LEPIRUDIN, which ↓es clearance by kidney- drug stays active longer-↑ anticoagulation). NO HIT! HIT, BIVALIRUDINangioplasty Inhibits VKOR (epoxide reductase), which normally regenerates vit K. ↓ synthesis of vit K dpd clotting factors II, VII, IX, & X. No- crosses placenta TRANSITION TO HEPARIN. DRUG-DRUG INTERACTIONS Bleeding, flatulence/diarrhea, cutaneous necrosis from ↓ prot C, chondrodysplasia punctata & blood dxs in fetus/kids. Long term tx for DVT, for 2-6 mos following MI, & atrial fibrillation. Also rodenticides. Know the properties of agents that can reverse the actions of heparin & warfarin. Side note: Vit K is fat-soluble vit made by gut bacteria (K2). Synthesis of factors II, VII, IX, & X dpd on it. Also impt for bone mineralization. WARFARIN’S antidote is PHYTONADIONE (Vitamin K1- made by plants): Prevents hemorrhagic dxs in newborns Correct vit K deficiency in ICU patients or patients on many antibiotics Counteract an overdose of Warfarin o PHYTONADIONE acts as an antagonist, promoting reestablishment of normal clotting factor activity. o Takes 6-24 hrs HEPARIN’S antidote is PROTAMINE SULFATE: Highly basic peptide that combines ionically w/ HEPARIN. Lasts 2 hrs. Routinely done in surgery, can even act as an anticoagulant. Anaphylaxis can occur in diabetic patients. 4. Understand how particular disease states &/or co-administration of other drugs alter the efficacy & side effects of warfarin (esp. why it is the metabolism of S-warfarin that is critical). Be able to describe specific pharmacokinetic & pharmacodynamic principles governing the interactions of warfarin, & be able to interpret specific examples. S-WARFARIN is the active form. Other drugs can affect the CYP2C9 of S-WARFARIN, while R-WARFARIN (CYP1A2, 2C19, 3A4) affects other drugs. Most importantly, if other drugs increase the effects of S-WARFARIN, we must be careful because of WARFARIN’S very low therapeutic index. Drugs/dxs that increase effects of WARFARIN do so by: inhibition of CYP450 metabolism (acute alcohol, liver dx) decreased plasma protein binding (phenytoin initially) decreased platelet/clotting factor fxn (antiplatelet drugs, HEPARINs, DTIs) decreased availability of vit K (diarrhea, inadequate diet) polymorphism in CYP2C9 slower metabolism Drugs/dxs that decrease effects of WARFARIN do so by: Induction of CYP450 metabolism (chronic alcohols, barbs, phenytoin ultimately) Decreased absorption (cholestyramine) Increased clotting factor/vit K conc (diuretics, hyperlipidemia, edema, hypothyroidism) Genetic defects in coagulation cascade such as Factor V Leiden & mutations in VKOR resistance to WARFARIN Drugs that ALWAYS interact w/ WARFARIN: aspirin, cimetidine, & phenytoin 5. Be able to use cytochrome p450 interaction tables to define drug-drug interactions (see the illustrative case on the Web). Inhibitors & substrates INCREASE the effectiveness of another drug metabolized by that same isozyme. Inducers DECREASE effectiveness. The ONLY affect of WARFARIN has on other drugs is to increase the plasma concentration bc neither SWARFARIN or R-WARFARIN are inducers or inhibitors of the CYP450 isozyme. Substrates can affect substrates only (like the previous scenario w/ S&R-WARFARIN. Inducers & inhibitors affect drugs in substrate column. Remember: all affect substrate. Inhibitor Inducer Substrate Substrates Inhibitor Inducer ↑Substrate serum conc & efficacy ↑Substrate serum conc & efficacy ↓Substrate serum conc & efficacy Many affects of drugs on WARFARIN: Substrates compete for binding sites increasing serum concs of both drugs (↓metabolism) leading to increased efficacy. (This is how WARFARIN works) Case Online: 1 drug as an inhibitor, simply inhibits the isozyme (eg. Fluvoxamine inhibits CYP2C9 for eg) resulting in increased serum substrate (S-WARFARIN) concentrations (↓ metabolism) leading to increased efficacy. 1 drug as an inducer, simply increases the activity of the isozyme (CYP2C9 for eg) resulting in decreased serum concentrations of that drug (↑metabolism) leading to decreased efficacy, while increasing the other drug serum concentrations (substrate: S-WARFARIN) thereby increasing its efficacy. – I am waiting for Dr. Fitz response about this! All three of these drugs are substrates, rather than inducers or inhibitors, of CYP450 isozymes: S-warfarin (the active ingredient) for CYP 2C9 R-warfarin for CYP 1A2, 2C19 and 3A4 metoprolol for CYP 2D6 amitryptylline for CYP 1A2, 2C19 and 2D6 So: Once physician changed to Fluvoxamine: amitryptylline and metoprolol compete with each other for metabolism via 2D6 R-warfarin increases the efficacy of amitryptylline via competition for 1A2 and 2C19 (amitryptylline doesn't affect the efficacy of warfarin because R-warfarin is not the active ingredient) Presumably, these interactions have been managed in this patient by adjusting dosages of all 3 medications, as evidenced by the fact that she has been stable for several years Once Paraxetine is added: Paroxetine is another substrate of CYP 2D6. Adding paroxetine increases the circulating concentrations of amitryptylline and metoprolol by decreasing their metabolism. Although amitryptylline is being given to this patient to manage her diabetic neuropathy, it is also a sedating antidepressant. Fluvoxamine is an extremely potent inhibitor of CYP 1A2 and 2C9, as well as 2C19 and 3A4. The most critical drug interaction caused by the addition of fluvoxamine is a decrease in the metabolism of S-warfarin (2C9), which is reflected in the increase in the patient's INR. The target range for INR is 2.5-3.5; a value of 5 means that this patient has severely decreased clotting factor concentrations, and is at risk for hemorrhage (actually, is already hemorrhaging, as evidenced by the dark urine). In addition, inhibition of 1A2 and 2C19 by fluvoxamine means that the amitriptyline concentrations will remain elevated (causing the sedation and dizziness), as well as increasing R-warfarin concentrations which will exacerbate the problems with amitriptyline The drug interaction tables would predict, therefore, that adding paroxetine would increase the side effects of amitryptylline, which include sedation and antimuscarinic effects (dry mouth, dizziness). 6. Know the specifics of the anti-coagulant, fibrinolytic & anti-inflammatory actions of DROTECOGIN ALFA. (USED IN DIC) Anti-coagulant actions: ↓ Va & VIIIa therefore ↓thrombin formation, ↓platelet formation & TF expression. Fibrinolytic actions: ↓TAFI & PAI-1 (both of which are inhibitors) allowing for ↑tPA (lysis of clot) Anti-inflammatory actions:↓TNF, ↓neutrophil activation, &↓cytokines from macrophages 7. Be able to describe w/ the biochemical mechanisms of action & adverse effects of antiplatelet agents. Know the specific instances where antiplatelet drugs are used in conjuction w/ other anti-clotting agents. Mechanism of action: Adverse Effects: ASPIRIN inhibits cyclooxygenase preventing PG synthesis. ABCIXIMAB, EPTIFIBATIDE, & TIROFIBAN block Gp IIb/IIIa receptor TICLOPIDINE & CLOPIDOGREL inhibit the binding fibrinogen to activated platelets by blocking the ADP receptor. DIPYRIDAMOLE inhibits cyclic nucleotide phosphodiesterase. (Ultimately, an inhibition of PDE33 - which is an inhibitor of cAMP2 - which normally inhibits degranulation1) (↓granulation3 - ↑ granulation2 - ↓ granulation1) 1-first step, 2-second step, 3-third step ABCIXIMAB, EPTIFIBATIDE, & TIROFIBAN + HEPARIN & ASPIRIN: percutaneous transluminal coronary angioplasty to prevent abrupt closure of vessel. DIPYRIDAMOLE + WARFARIN: prevents embolization from prosthetic heart valves ASPIRIN bleeding, ulcers, CNS, tinnitus ABCIXIMAB (hypersensitivity-TB), EPTIFIBATIDE, & TIROFIBAN bleeding & thrombocytopenia TICLOPIDINE (most) & CLOBPIDOGREL bleeding, diarrhea, rash (10-15%); leucopenia (1%); & thrombotic thrombocytopenia purpura (<1%). DIPYRIDAMOLE neglegible 8. Know the drugs & plasma fractions that are used specifically in the tx of vit K deficiency, factor VIII deficiencies [classic hemophilia (hemophilia A); and von Willebrand’s disease] and factor IX deficiency (Christmas disease, hemophilia B). Deficiency/Dx Vit K (newborns & ICU/multiple antibiotics patients) Hemophilia A & von Willebrand’s dx (Factor VIII) Hemophilia B, Christmas dx (Factor IX) Drug/plasma fraction PHYTONADONE LYOPHILIZED Factor VIII, CRYOPRECIPITATE (Factor VIII & vWf), & DESMOPRESSIN ACETATE (↑es Factor VIII in mild cases) (FACTOR IX) 9. Understand the mechanism of action of the fibrinolytic inhibitors aminocaproic acid & aprotinin. AMINOCAPROIC ACID (EACA) inhibits plasminogen activation (counteract tPAs) APROTININ is a serine protease inhibitor of the plasmin-streptokinase complex, thereby preventing fibrinolysis.