Supplementary Legends - Word file (63 KB )
advertisement

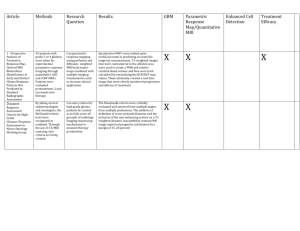
LEGENDS TO SUPPLEMENTARY TABLES The supplementary tables below summarize the data concerning the effect of saturating concentration of BMP4 (100 ng/ml) on cells isolated from human GBMs. The cell preparations shown here were established as follows: 1. GBSC-1: specimen from an adult GBM, sorted for their positive staining with the anti-CD133 antibody and briefly cultured (48 hours) in the presence of EGF and FGF2 1. Data from this preparation are also presented in the main manuscript. 2. GBSC-2: specimen from an adult GBM, sorted for their positive staining with the anti-CD133 antibody and briefly cultured (48 hours) in the presence of EGF and FGF2 1. 3. GBSC-3: cells isolated from a specimen of adult GBM, unsorted and briefly cultured (48 hours) in the presence of EGF and FGF2 2. 4. Acutely Dissociated Cells-1: Cells analyzed soon after the same enzymatic/mechanical dissociation used for GBSCs above. 5. Acutely Dissociated Cells-2: Cells analyzed soon after the same enzymatic/mechanical dissociation used for GBSCs above. Supplementary table 1a – analysis of the phosphorylation of Smad proteins by BMP4 The data show a significant increase in the levels of phosphorylation of smad 1,5,8 proteins (as shown in figure 1, panels h through l) upon exposure of the different cell preparations to BMP4 for 90’ minutes as evaluated by cytofluorimetric analysis (* p< 0.01, n=3, two-tailed Student’s t-test). Supplementary table 1b, c and d – analysis of apoptosis, cell death and proliferation in BMP4-treated GBM cells. Upon 48-hour exposure to BMP4 neither cell death (table 1b) nor apoptosis (table 1c) were significantly changed. Conversely, the same treatment triggered significant loss of proliferation capacity as indicated by a significant decrease in the KI67 labelling index in all cell preparations (table 1d) (* p< 0.05, n=3, two-tailed Student’s t-test). Supplementary table 1e – Analysis of the cell cycle following exposure of GBM cells to BMP4 In all cell preparations, a 48-hour exposure to BMP4 consistently elicited an increase in the percentage of cells in the G1 phase of the cell cycle, accompanied by a concomitant decrease of the percentage of cells in S phase (*p<0.05; **p<0.001, n=3, two-tailed Student’s t-test). Supplementary table 1f and 1g – BMP4 decreases both the clonogenic index and percentage of CD133+ cells. A 48-hours exposure to BMP4 significantly decreased both the clonogenic frequency (table 1f) and the size of the CD133+ population (table 1g) in all of the GBM preparations tested (* p<0.05, ** p<0.01 n=3, for briefly cultured cells and *p<0.05, ** p<0.01 n=1 in triplicate, for acutely dissociated cells, two-tailed Student’s t-test). Supplementary table 1h - phenotype analysis on GBM cells Similar to the data shown in fig. 3 panels m through r, the table shows the results from quantitative flow cytometry analysis of the changes in the levels of expression of neural lineage specific markers in cells from human GBMs following a 48 hours exposure to BMP4 (in the continued presence of mitogens). A major increase is observed in the expression of the astroglial marker GFAP in all cell preparations, together with a consistent increase in the neuronal III-tubulin (TUJ1) or TAU1 antigens and the oligodendroglial marker GalC. Data are from one representative experiment, out of three, giving similar results. Supplementary table 1i - phenotype analysis on GBM cells A 48-hour exposure to BMP4 caused a significant increase in the number of MAP5immunoreative (IR) cells displaying a mature phenotype as established by counting all MAP5-IR cells with processes at least three times longer that the diameter of the soma. (* p< 0.05, n=3, two-tailed Student’s t-test). LEGENDS TO SUPPLEMENTARY FIGURES Supplementary Figure 1 – The figure shows a quantitative analysis (Real Time PCR (RT-PCR)) of the expression of the BMPR type 1A, 1B, and 2 genes in the different GBM cell preparations investigated in this study – acutely dissociated or briefly cultured (GBSC) cells. The analysis confirmed a lower, though variable level of expression for all the BMPRs in cells from GBMs with respect to normal human foetal neural stem cells (HFNSCc). Values shown are representative of three different experiments (*p<0.001 vs HFNSCs, n=3, two-tailed Student’s t-test). Supplementary Figure 2 – a. Dose response curves showing the ability of BMP4 to inhibit the proliferation of two representative GBM cell preparations (one established from CD133+ sorted (blue line) and one from unsorted cultures (purple line)) in a dose-dependent fashion, at a saturating concentration of 100ng/ml. b. Cytofluorimetric analysis (one representative plot of three independent experiments yielding similar results) showing the phosphorylation and nuclear translocation of phospho Smad 1,5,8 as elicited 1.5 hours post BMP4 treatment in cultured GBM cells (left panel). No activation of the p38 MAPK pathway (right panel) was seen 1.5 hours after BMP4 treatment (similar plots were seen at 15 minute intervals, up to 2 hours; control-red, BMP4-green, dotted line-isotype control). See also supplementary table 1a for complete, quantitative data. Supplementary Figure 3 – Effects of various BMPs (all at 100 ng/ml) on the growth of GBM cells. BMP2, -4,-5,-6,-7,-8b inhibit cell growth, whereas BMP1, -3 and -3b appear to be ineffective, similar to TGF1 and 2 and to TGF1.2 (a chimerical TGF agonist polypeptide) (all at 100 ng/ml), which were also ineffective.* p<0.005 BMPs vs control, mean ± SE n=3, two-tailed Student’s t-test; ** p<0.001 BMP4 vs control, mean ± SE n=3, two-tailed Student’s t-test. Supplementary Figure 4 – a. The figure shows the effect of BMP4 on the expansion rate of briefly cultured GBM cells (GBSC-2, pre-sorted for CD133+, black lines; GBSC-3, unsorted, red lines) expanding in the presence of mitogens. BMP4 inhibits cell expansion in both preparations (mean±SE, n=3; p<0.005) b. Representative plots portraying the relative percentage of cells engaged in the various phases of the cell cycle in control and BMP4-treated GBM cells. This was investigated by analyses of the DNA content per cell, using propidium iodide incorporation followed by flow cytometry. The data demonstrate that BMP4 treatment (right) results in a significant increase of the proportion of cells in G0/G1 with a corresponding decrease in the percentage of cells in S phase as compared to control cells (left). Complete quantitative analysis in supplementary table 1e. c. Relative to control (left panel) a more differentiated phenotype is seen upon exposure to BMP4 (second panel from left) of GBM cells plated onto Matrigel. Consistent with the concept that BMP4 induces a more differentiated phenotype, a subjective more differentiated morphology and an increase in the expression of MAP5-immunoreactivity is seen in BMP4-treated (right panel) as compared to control (second panel from right) GBM cells (see also supplementary table 1i for quantitative analysis). Supplementary Figure 5 – The figure shows one example of the dot plot graph concerning the quantitative analysis of the expression of the CD133 antigen. This was performed by flow cytometry on control (b and isotype control in a) and BMP4- treated GBM cells (d and isotype control in c). It is clear that BMP4 triggers a significant decrease in the actual overall percentage of CD133-immunoreactive cells. Supplementary Figure 6 – Untreated GBM cells constitutively express neuronal markers such as MAP2 (a) and NF200 (c) as well as the intermediate filament nestin, normally found in immature neural precursor, (e). These markers are still expressed after 48-hour exposure to BMP4 (b, d and f, respectively). a-f. Scale 15µm, as in f. Supplementary Figure 7 – BMP4 inhibits the tumorigenicity of GBM cells. Transient (48 hours) exposure of cultured GBM cells to BMP4 in vitro, prior to transplantation into the striatum of adult SCID/bg mice, caused a dramatic reduction in the ability of the implanted cells to form tumors (b,d,f,h,j,l) as compared to controls (a,c,e,g,i,k). Hematoxylin and eosin staining showed typical glioblastoma masses five weeks after injection of control cells (a,e,g), whereas BMP4-treated cells generated very small grafts (b,f,h). Tumors established from control cells displayed a much higher mitotic index, as revealed by immunocytochemistry for Ki67 (4.3 ± 0.3% control vs. 0.76 ± 0.5% BMP treated, mean ± SE, n=3, Student’s t-test, p<0.05) (c, high power in i), than BMP4-treated cells (d, high power in j). k,l. Hematoxylin and eosin staining showing invasion of the lumen of the right lateral ventricle at bregma point (k, arrow) five weeks post-injection) following implantation of control cells. This phenomenon was never observed with BMP4-pretreated GBM cells (l, arrow). Three months post-injection, all control animals had died, whereas all animals receiving BMP4 pre-treated cells survived well beyond 5 months. m,n. Following a 48 hours incubation in control conditions (control), CD133+ cells were purified by FACS (purity 97.3%) and the ability of 3 x 105 of these cells to establish GBMs intracerebrally was compared to that of the same number of CD133+ cells, purified from sister cultures (purity 98.7%) after incubation for 48 hours in the presence of BMP4 (treated). Mice transplanted with CD133+ cells from control cultures developed large neoplastic formations by 5-7 weeks post transplant (m; n=8), whereas none of the animals (n=8) receiving CD133+ cells sorted from BMP4-treated cells developed any tumor (n). By ninety days post-injection all of the control animals had died whereas those receiving CD133+ cells from BMP4-treated cultures were alive. This shows that the tumorigenic ability of the residual fraction of CD133+ cells found in BMP4-treated cultures is virtually abolished by BMP4. o,p. When CD133+ cells, that were acutely isolated from primary tumors, established in mice transplanted with CD133+ acutely isolated control cells, were re-transplanted into the brain of secondary recipients they established secondary tumors in less than 4 months (example in o). These same cells could also be cultured and expanded with mitogens (see main text). Neither culturing nor establishment of secondary tumors could be accomplished with cells extracted from mice, which were initially transplanted with BMP4-treated, acutely isolated GBM cells. Similar serial transplantation experiments yielded equivalent results when the primary tumor was generated by transplantation of 3 x 105 briefly cultured CD133+ cells (p; example of secondary tumor established from CD133+ briefly cultured cells). Magnification: a-h and k-n, 5X; i,j and o,p, 10X. Supplementary Figure 8 – Ex vivo pre-treatment of GBM cells with BMP4 inhibits ventricular invasion following orthotopic injection. Five weeks after transplantation into the adult brain of scid/bg mice, tumors from control GBM cells have invaded the ventricular system (see also supplementary fig.7) including the fourth ventricle (a). Cells from cultures pretreated with BMP4 for 48hours showed no signs of ventricular invasion (b). Magnification: 5X. Supplementary Figure 9 – a,c. Phenotype analysis confirmed that, as shown previously 2, tumors induced by transplanting briefly cultured GBM cells into the brain of scid/bg animals generated astroglial-like cells, (shown by GFAP-IR (green and anti-human mitochondria [red in a] or anti-human nuclei immunofluorescence staining [red in c]). Within the tumor mass, numerous cells were also nestin-IR (intermediate filament found in immature neural precursors; e, magnified in g, brown staining). No labelling with neuronal markers, such as III-tubulin or NF200, or oligodendroglial antigens, such as GC or O4 was observed. Scarce GFAP- (b,d) and nestin-IR (f,h) were also observed in BMP4-treated GBM cell implants. When, as in the case of BMP4 co-treatment of GBM cells during transplantation (b,f), tumor development was poor, the staining for GFAP revealed scattered reactive astroglial cells (green in b) surrounding the transplantation area (red, anti-human mitochondria in b), wherein a few surviving GBM cells were weakly stained with the anti-nestin antibody (f,h). In all the BMP4 treatment paradigms, the tumors contained GFAPimmunoreactive cells (d), but no labelling with neuronal or oligodendroglial marker was observed. Magnification: a,b and e,f 5X; c and d 20X; g and h, 40X. Supplementary Figure 10 – a. Quantitative RT-PCR analysis of the expression of thirteen different BMPs genes in briefly cultured GBM cells. Data are the mean ± se samples in triplicate from one out of three independent experiments yielding overlapping results. b. When GBM cells, cultured with mitogens, were exposed to a neutralizing antibody (nAb-BMP4; 10µg/ml, R&D Systems) that specifically blocks BMP4, the cell growth rate increased significantly as compared to control conditions (*p<0.01 nAb-BMP4 vs control). This indicates that endogenous BMP4 (RT-PCR and western blot in Fig. 1) physiologically restricts the proliferation of human GBM cells. c. The normal levels of phosphorylation of smad 1,5,8 proteins observed in control cells is almost halved following a 3 hours exposure to 10µg/ml of neutralizing anti-BMP4 antibody, whereas exposure to BMP4 produces the usual increase, as described earlier on Fig.1. Evaluation by cytofluorimetric analysis (* p< 0.01, n=3, two-tailed Student’s t-test). REFERENCES 1. 2. Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432, 396-401 (2004). Galli, R. et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 64, 7011-21 (2004).