tpj12907-sup-0012-MethodS1
advertisement

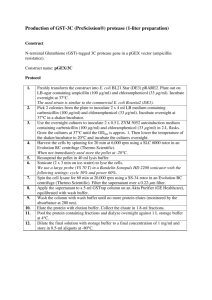
1 1 Supporting experimental procedures 2 3 Organelle-Specific Fluorescent Dye Staining 4 For observation of root cells, GFP-DRP1E transgenic plants (GFP-DRP1Eox) were grown on 5 half-strength MS medium with 1% (w/v) sucrose and 1% (w/v) agar for 10 days. To visualize the 6 plasma membrane, roots were stained with 8 μM FM4-64 (Invitrogen). To stain mitochondria, 7 roots were stained with 500 nM MitoTracker Red CMXRos (Invitrogen) for 20 min at room 8 temperature and then washed three times with distilled water. Root cells were observed at room 9 temperature using a microscope (BX-61; Olympus) with the confocal laser scanning unit of a CSU 10 10 (Yokogawa), a 488-nm laser (Furukawa) and a 561-nm laser (Cobolt AB, Stockholm, Sweden). 11 Images were captured with the image analysis software SlideBook (Intelligent Imaging 12 Innovations). 13 14 15 Two Dimensional Differential Gel Electrophoresis (2D-DIGE) 2D-DIGE analysis was performed according to the method described previously 16 (Minami et al., 2009). Briefly, plasma membrane fractions cleaned with a 2D-Clean-up kit (GE 17 Healthcare) were resuspended in a lysis buffer [7 M urea, 2 M thiourea, 2% (w/v) ABS-14, 0.5% 18 (w/v) Triton X-100 and 10 mM Tris-HCl (pH 8.5)]. 2D-DIGE analyses were then carried out 19 according to the manufacturer’s CyDye DIGE Fluors protocol (minimal dyes, GE Healthcare). 20 Protein samples (20 µg proteins) from wild-type or drp1e mutant plants were minimally labeled 21 with either Cy3 or Cy5 dye. The IPG strips were rehydrated at 20°C for 12 h at 100 V after 22 application of the protein samples and then isoelectrofocused at 20°C in the IPGphor system with 23 a successive increase in voltage [500 V (1 h), 1,000 V (1 h), 2,000 V (1 h), 4,000 V (1 h), 5,000 V 2 24 (1 h), 6,000 V (2 h) and 8,000 V (7 h)]. After isoelectrofocusing was completed, gel strips were 25 subjected to 10% SDS-PAGE as described above. Two biologically independent samples and two 26 technical replications of each sample were performed. Quantitative analysis was performed 27 according to the method described previously (Minami et al., 2009). 28 29 30 Purification of GST-Fused DRP1E Protein DRP1E cDNA cloned into pENTR/D-TOPO was transferred into the pDEST15 vector 31 containing GST tags with Gateway technology. The pDEST15-DRP1E plasmid was transformed 32 into the E. coli strain BL21 (DE3) and expression of the GST-fused DRP1E protein was induced by 33 incubating the cells overnight with 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) at 20°C. 34 The bacterial cells were lysed with 50 µg/mL lysozyme and sonication. After centrifugation, the 35 supernatant was used to purify recombinant GST-DRP1E protein using Poly-prep 36 Chromatography columns (Bio-Rad Laboratories) containing glutathione-sepharose 4B (GE 37 Healthcare) according to the manufacturer’s protocol. Because the recombinant GST-DRP1E was 38 not eluted under normal conditions, we used 1.5 M NaCl buffer containing 50 mM Tris-HCl (pH 39 10.0), 50 mM reduced glutathione, 1.5 M NaCl and EDTA-free protease inhibitor cocktail (Roche 40 Applied Science, Basel, Switzerland) to elute the recombinant protein. Recombinant His-GST 41 protein was used as a control and purified using a standard protocol. Purified protein was dialyzed 42 against 20 mM Tris-HCl and 500 mM NaCl and used for the protein-lipid overlay analysis. 43 44 45 46 Protein-Lipid Overlay Assay The in vitro lipid-binding assay was performed as described by Kim et al. (2001). Lipids were obtained as follows: PA, PC, PI and PS from Doosan Serdary Research Laboratories 3 47 (Etobicoke, Canada); PE and PG from Avanti Polar Lipids (Alabaster, AL); cholesterol from Wako 48 Pure Chemical Industries (Osaka, Japan); plant glucocerebroside (GlcCer) from Matreya 49 (Pleasant Gap, PA); and PI(3)P, PI(4)P and PI(4,5)P from Echelon Biosciences (Salt Lake City, 50 UT). In all cases, 4 µL lipids (equivalent to 0.8 ng) were dissolved in chloroform:methanol:water 51 (1:2:0.8, v/v/v) and spotted onto nitrocellulose membranes (Hybond-C Extra; GE Healthcare UK 52 Ltd, Little Chalfont, UK). The membranes were blocked with a solution consisting of 20 mM 53 Tris-HCl (pH 7.5), 150 mM NaCl and 0.1% (w/v) Tween 20 (TBS-T) overnight at 4°C and then 54 incubated with 0.5 µg/mL purified recombinant protein in TBS-T containing 3% (w/v) BSA for 1 h at 55 room temperature. After washing with TBS-T, the membrane was incubated with an anti-GST 56 (mouse IgG2aκ) monoclonal antibody (Nacalai Tesque) for 2 h at room temperature. After 57 re-washing with TBS-T, the membrane was incubated with a 1/2000 dilution of horseradish 58 peroxidase (HRP)-conjugated goat anti-rabbit or anti-mouse IgG (H+L) secondary antibodies 59 (Thermo Fisher Scientific, Waltham, MA) and washed again. The signal was detected using 60 Super-Signal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL) in a Light Capture 61 system (ATTO, Tokyo, Japan). 62 63 Identification of DRP family proteins in co-immunoprecipitate fractions of GFP-specific 64 antibody using microsomal fractions of DRP1E-GFPox seedlings 65 One mg of microsomal fractions from DRP1E-GFPox and wild-type (control) plants were 66 homogenized in buffer (10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 2 mM EDTA, 0.5% Nonidet P-40 67 (v/v) and protease inhibitors) and incubated with anti-GFP antibody-coated Dynabeads 68 (Invitrogen) overnight at 4˚C. After the samples were washed five times with TBS buffer, the 69 immunoprecipitations were eluted by SDS sample buffer. Nano-LC−MS/MS (LTQ Orbitrap XL, 4 70 Thermo Fisher) analysis according to Li et al. (2012a) and Takahashi et al. (2012) was used for 71 identification of proteins in IP fractions.