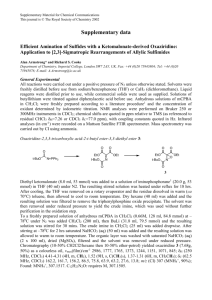
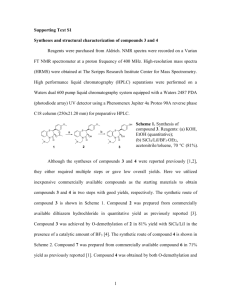
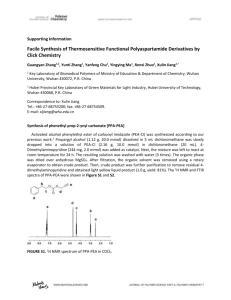
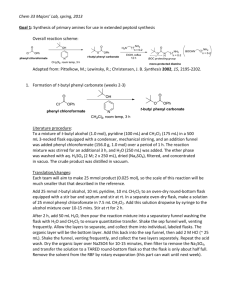
Electronic Supplementary Materials Preparation of Stimuli
advertisement

Electronic Supplementary Materials Preparation of Stimuli-Responsive Hydrogel Networks with Threaded βCyclodextrin End-Capped Chains via Combination of Controlled Radical Polymerization and Click Chemistry Tao Cai,a Wen Jing Yang,a Zhengbiao Zhangb, Xiulin Zhu*,b, Koon-Gee Neoha,c and En-Tang Kang*,a,c aNUS Graduate School for Integrative Science and Engineering National University of Singapore Kent Ridge, Singapore 117576 bSchool of Chemistry & Chemical Engineering Soochow University Suzhou, PR China 215006 cDepartment of Chemical & Biomolecular Engineering National University of Singapore Kent Ridge, Singapore 119260 *To whom correspondence should be addressed Email address: xlzhu@suda.edu.cn (XL Zhu); cheket@nus.edu.sg (ET Kang) S1 Figure S1. XPS wide scan, C 1s and N 1s core-level spectra of the azido-βCD powder. Other materials Acryloyl chloride (97%), 4-(N,N-dimethylamino)pyridine (DMAP, ≥99%), poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (Pluronic® F-108, PEG-b-PPG-b-PEG, 82.5 wt% of PEG, Mn=14,600 g/mol), triethylamine (TEA, ≥99%), N-ethyl-N'-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDAC, ≥99%) and propargyl alcohol (PA, 99%) were obtained from Sigma-Aldrich Chem. Co. and used as received without further purification. Dichloromethane (DCM, reagent grade) was obtained from Merck Chem. Co. and used as received without further purification. S,S'bis(α,α'-dimethyl-α''-acetic acid)trithiocarbonate (BDAT)1,2 was prepared according to procedures described in the literature. Synthesis of S,S'-bis(α,α'-dimethyl-α''-propargyl acetate)trithiocarbonate (BDPT) S,S'-bis(α,α'-dimethyl-α''-propargyl acetate)trithiocarbonate (BDPT) was prepared according to the method reported in literature with slight modification.3-5 BDAT (0.50 g, 1.77 mmol), EDAC (1.02 g, 5.3 mmol), DMAP (0.65 g, 5.3 mmol) and anhydrous S2 CH2Cl2 (10 ml) were introduced into a 25 ml single-necked round bottom flask. The flask was immersed in an ice bath, and propargyl alcohol (0.5 ml, 8.6 mmol) in 5 ml of dry CH2Cl2 was added drop-wise. Upon completion of the addition, the solution was stirred at room temperature for 24 h. The product was washed with doubly distilled water and brine several times. The combined organic layer was dried over magnesium sulfate and concentrated by rotary evaporator. About 0.45 g of a yellow liquid was obtained (yield ~71%). 1H NMR (CDCl3, δ, ppm, TMS): 1.67 (12H, -CH3), 2.46 (2H, -C≡CH), 4.68 (4H, -COOCH2-). Synthesis of PEG-b-PPG-b-PEG Diacrylate6 In a typical reaction, PEG-b-PPG-b-PEG (7.3 g, 0.5 mmol), TEA (0.25 g, 2.5 mmol) and CH2Cl2 (20 ml) were introduced into a 50 ml single-neck round-bottom flask. The reaction flask was immersed in an ice bath at 0 ºC. Acryloyl chloride (0.23 g, 2.5 mmol) in 5 ml anhydrous CH2Cl2 was added dropwise to the flask. After the addition, the flask was sealed, and the mixture was left to react at room temperature for 24 h. At the end of the reaction, the mixture was filtered to remove the precipitated salt and the filtrate was precipitated in an excess amount of anhydrous diethyl ether. The precipitated copolymers were purified by re-dissolving in CH2Cl2 and re-precipitation in diethyl ether. The dissolution-re-precipitation cycle was repeated twice. The purified product was dried under reduced pressure at room temperature. About 7.0 g of a white solid was obtained (yield ~95%). 1H NMR (CDCl3, δ, ppm, TMS): 1.12 (methyl group of PPG), 3.4 (methylidyne group of PPG), 3.55 (methylene group of PPG), 3.65 (methylene group of PEG), 4.3 (4H, -COOCH2-), 5.8 (2H, CH2=CH), 6.15 and 6.4 (4H, CH2=CH). S3 a S c O O S S O O b a b c (b) a S HO OH S a S O O 4 3 (a) 6 5 2 1 0 Chemical shift (ppm) Figure S2. 1H NMR spectra of (a) S,S'-bis(α,α'-dimethyl-α''-acetic acid)trithiocarbonate (BDAT) and (b) S,S'-bis(α,α'-dimethyl-α''-propargyl acetate)trithiocarbonate (BDPT). S4 d O O a b O O m c n O p f O g e a d O c b g f (b) HO O e d a b d O m O c n O p OH a c b (a) 7 6 5 4 3 2 1 0 Chemical shift (ppm) Figure S3. 1H NMR spectra of (a) PEG-b-PPG-b-PEG and (b) PEG-b-PPG-b-PEG diacrylate block copolymers. S5 (e) (d) (c) Exo. (b) 39.8 ºC 42.6 ºC 45.1 ºC 55.4 ºC (aʹ) Endo. 56.1 ºC (a) 58.6 ºC 20 40 60 Temperature (℃) 80 Figure S4. Typical DSC thermograms of the (a) primary PEG-b-PPG-b-PEG, (b) slidingring PEG-b-PPG-b-PEG-thread-βCD, (c) sliding-ring PEG-b-PPG-b-PEG-thread-βCDcapped-PMES36, (d) sliding-ring PEG-b-PPG-b-PEG-thread-βCD-capped-PMES53 and (e) sliding-ring PEG-b-PPG-b-PEG-thread-βCD-capped-PMES68 hydrogels, and (a′) PEG-bPPG-b-PEG and βCD-capped-PMES53 (1:1, w/w) blend hydrogels. S6 References [1] J. T. Lai, D. Filla, R. Shea, Macromolecules, 2002, 35, 6754-6756. [2] S. H. Thang, Y. K. Chong, R. T. A. Mayadunne, G. Moad, E. Rizzardo, Tetrahedron, 1990, 40, 2435-2438. [3] T. Zhang, Z. H. Zheng, X. B. Ding, Y. X. Peng, Macromol. Rapid Commun., 2008, 29, 1716-1720. [4] L. Wang, K. Zeng, S. X. Zheng, ACS Appl. Mater. Interface, 2011, 3, 898-909. [5] X. M. Lian, J. Jin, J. Tian, H. Y. Zhao, ACS Appl. Mater. Interface, 2010, 2, 22612268. [6] F. Cellesi, N. Tirelli, J. A. Hubbell, Macromol. Chem. Phys., 2002, 203, 14661472. S7