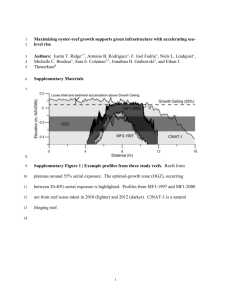
Temperate reefs - Department of Environment, Land, Water and
advertisement

Seagrass and Reef Program for Port Phillip Bay: Temperate Reefs Literature Review No. 11 May 2010 Seagrass and Reef Program for Port Phillip Bay: Temperate Reefs Literature Review Neil Hutchinson, Taylor Hunt and Liz Morris May 2010 Fisheries Victoria Department of Primary Industries If you would like to receive this information/publication in an accessible format (such as large print or audio) please call the Customer Service Centre on: 136 186, TTY: 1800 122 969, or email customer.service@dpi.vic.gov.au Author Contact Details: Neil Hutchinson Fisheries Research Branch, Fisheries Victoria PO Box 114, Queenscliff Victoria 3225 Copyright The State of Victoria, Department of Primary Industries, 2010. Copies are available from the website: www.dpi.vic.gov.au/fishing This publication is copyright. No part may be reproduced by any process except in accordance with the provisions of the Copyright Act 1968. Preferred way to cite: Hutchinson, N., Hunt, T., and Morris, L. (2010). Seagrass and Reef Program for Port Phillip Bay: Temperate Reefs Literature Review. Fisheries Victoria Technical Report No. 11, 61 pages. Department of Primary Industries, Queenscliff, Victoria, Australia. ISSN 1835-4785 ISBN 978-1-74264-141-6 (print) Temperate Reefs literature review ii Authorised by the Victorian Government, 2a Bellarine Hwy, Queenscliff, Victoria 3225 Printed by DPI Queenscliff, Victoria Published by the Department of Primary Industries. General disclaimer This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication. Executive Summary The purpose of this document is to review the existing scientific understanding, in an international context, of the temperate reef habitats in Victoria, particularly in Port Phillip Bay. A variety of research has been conducted on intertidal, subtidal, deep subtidal and canyon reefs in Victoria both within Port Phillip Bay and along the Victorian coast. While some clear differences in species distributions and assemblage structure have been identified from eastern to western Victoria, the majority of information on reefs in Victoria provides only a “snapshot” at one particular place or time of year. Examination of temporal and spatial variation in the structure of assemblages on different reef types, and more basic information on which species occur and their basic ecology and behaviour are lacking, especially for deep and canyon reefs. More information is required to enable us to understand what is shaping many of Victoria’s reef communities. There is a need to conduct research that examines Victoria’s marine ecosystems in a broader geographical context across southern Australia, and also in the context of coastal oceanography. The amount of research and level of understanding has been greatest for intertidal reefs, is lower for subtidal reefs, and is very limited for deep and canyon reefs. This is probably a reflection of ease of accessibility and logistical constraints. Research on subtidal reefs in Port Phillip Bay has been fragmentary, and there is a poor understanding of the drivers influencing the reef communities and how these differ from the open coast. Further research on the physiological and ecological drivers affecting subtidal reefs in Port Phillip Bay is required. Of special interest are the deep reef and canyon habitats of Port Phillip Heads. This area has a unique combination of deep water, strong currents, and coastal sedimentary processes. The sessile invertebrate communities are well developed but their uniqueness is uncertain. Detailed studies of taxonomy and assemblage structure are required along with research into the physical and ecological processes affecting the system. Temperate reefs literature review iii Table of Contents Executive Summary............................................................................................. iii Introduction ............................................................................................................ 1 Temperate reefs ......................................................................................................................................................... 1 Key reef types in Victoria ........................................................................................................................................ 1 1. Intertidal .......................................................................................................................................................... 1 2. Subtidal ............................................................................................................................................................ 1 3. Deep: ................................................................................................................................................................ 1 4. Canyon: ............................................................................................................................................................ 1 Biogeographic patterns across Victoria ............................................................. 2 Oceanographic and productivity context of Victorian reefs......................... 5 Central Victoria ......................................................................................................................................................... 5 Western Victoria ........................................................................................................................................................ 5 Eastern Victoria ......................................................................................................................................................... 5 Upwelling ................................................................................................................................................................... 5 Current monitoring ............................................................................................... 7 Monitoring ................................................................................................................................................................. 7 Marine Habitat Classification ................................................................................................................................. 7 Ecosystem services ................................................................................................ 8 Physical habitats and biological communities ................................................ 9 Intertidal reefs ........................................................................................................................................................... 9 Subtidal reefs ........................................................................................................................................................... 11 Exposed subtidal reefs ......................................................................................................................................... 11 Sheltered subtidal reefs ....................................................................................................................................... 12 Deep reefs ................................................................................................................................................................. 14 Invertebrates ......................................................................................................................................................... 14 Fish ......................................................................................................................................................................... 15 Canyon reefs ............................................................................................................................................................ 17 Port Phillip Heads ................................................................................................................................................ 17 Other canyons ....................................................................................................................................................... 17 Temperate reefs literature review iv Ecological and physical environmental drivers ............................................ 19 Intertidal reefs .......................................................................................................................................................... 19 Top-down / disturbance processes ..................................................................................................................... 20 Bottom-up / supply side processes ..................................................................................................................... 22 Competition ........................................................................................................................................................... 24 Subtidal reef ............................................................................................................................................................. 25 Physical drivers ..................................................................................................................................................... 25 Ecological drivers .................................................................................................................................................. 27 Major commercial fisheries.................................................................................................................................. 30 Deep reefs and canyons .......................................................................................................................................... 32 Physical drivers ..................................................................................................................................................... 32 Ecological drivers .................................................................................................................................................. 33 Knowledge gaps ....................................................................................................................................................... 34 Intertidal reefs ....................................................................................................................................................... 34 Subtidal reefs ......................................................................................................................................................... 34 Deep reefs and canyons ....................................................................................................................................... 35 References ............................................................................................................. 37 Appendix 1 Tables............................................................................................... 51 Appendix 2 Conceptual Models ....................................................................... 56 Temperate reefs literature review v List of Figures Figure 1. Key relationships and drivers on intertidal reefs in Victoria............................................................. 57 Figure 2. Key relationships and drivers on subtidal reefs in Victoria............................................................... 58 Figure 3. Key relationships between invertebrates on subtidal reefs in eastern and western Victoria ........ 59 Figure 4. Key relationships and drivers on deep reefs in Victoria .................................................................... 60 Figure 5. Key relationships and drivers on canyon reefs in Victoria ................................................................ 61 List of Tables Table 1. Six bioregions relevant to Victoria as presented in Interim Marine Coastal Regionalisation of Australia Technical Group, IMCRA (2006) ................................................................................................... 51 Table 2. Interim intertidal marine habitat (MHC) categories for Victoria, Ferns et al. (2000). ....................... 52 Table 3. Interim shallow subtidal (0 – 2.5 m) marine habitat (MHC) categories for Victoria, Ferns et al. (2000). ................................................................................................................................................................. 52 Table 4. Primary shallow habitat classification scheme as presented in Ball et al. (2000). ............................. 53 Table 5. Potential physical drivers responsible for shaping deep reef assemblages in Victoria. Summarised from Edmunds et al. (2006b) ........................................................................................................................... 54 Table 6. Potential ecological drivers responsible for shaping deep reef assemblages in Victoria. Summarised from Edmunds et al. (2006b) .................................................................................................... 55 Temperate reefs literature review vi Introduction Temperate reefs Key reef types in Victoria Temperate reefs are defined here as hard substrata in marine waters averaging temperatures below 18C (The University of Queensland 2001; Port of Melbourne Corporation 2007c). They are widely distributed in Australia, and extend around southern Australia from Kalbarri in Western Australia to Noosa in Queensland. For the purposes of this literature review we have grouped reefs in Victoria into four broad categories: In Victoria, temperate reef habitats cover extensive areas of the coastline and are known for their high biological complexity, species diversity, species richness, level of endemism and productivity (Keough and Butler 1996; Edmunds et al. 2003). These habitats are of social and cultural value to people due to a variety of indigenous, aesthetic, recreational and historical aspects (Keough et al. 1990; Edmunds et al. 2006a). They also hold significant economic value by supporting two of the most valuable commercial fisheries in Australia, in abalone and rock lobster, plus other valuable activities such as recreational fishing, diving and a range of other tourism activities (Environment Conservation Council 2000; Edmunds et al. 2003; Department of Primary Industries 2009b). But, despite the great importance of temperate reefs, they are not well known scientifically (Keough and Butler 1996). The purpose of this literature review is to critically evaluate the current understanding of Victoria’s temperate reefs, with particular reference to reef communities, ecological and physical environmental drivers, dynamics, key structuring and ecosystem services, key relationships, and any key current relevant research/monitoring programs. The review will also construct conceptual models, summarizing key relationships and provide an indication of scientific uncertainty. 1. Intertidal Reef that is periodically exposed to air at low tide but is submerged or directly influenced by sea water at high tide. Intertidal reefs are also known as rocky shores. 2. Subtidal Reef that is never exposed to the air from tidal influences, generally covering depths of 2.5-20 m. The review will distinguish and discuss differences in communities and processes on subtidal reefs split into two categories based on wave exposure and currents: Exposed subtidal reefs found on the open coast and Port Phillip Heads Sheltered subtidal reefs found within Port Phillip Bay. 3. Deep: Reef that is located 20 m or deeper. 4. Canyon: Deep reef located in canyons (geomorphological formations). Particular attention will be given to the Port Phillip Heads deep reef canyon. These categories were selected as they represent reef types across Victoria. They distinguish reef types based on their exposure to key physical modifiers including depth, tidal range, energy (wave and currents) and photic influences. These factors have been shown to be fundamental drivers of the distribution patterns of benthic marine communities (O'Hara et al. 1999; Ferns et al. 2000; Ball et al. 2006; Coleman et al. 2007). Temperate reefs literature review 1 Biogeographic patterns across Victoria Scientific research on temperate reef communities in Victoria has, to some extent, focused on the classification of marine waters into bioregions. By examining qualitative measures of biological or physical variables at intertidal and subtidal sites across Victoria, broad-scale biogeographic patterns have been identified and subsequently supported in later studies. Early studies of intertidal and subtidal temperate reef communities along the coast of southern Australia categorised communities into three distinct marine biogeographic provinces (Bennett and Pope 1952; Dartnall 1974; Edgar 1984; Womersley and King 1990). These provinces were: The western, warm temperate, “Flindersian Province” The eastern, warm-temperate, “Peronian Province” The southern, cool-temperate, “Maugean Province” Edmunds et al. (2000) briefly reviewed these studies and surmised that the coast of Victoria is situated at the confluence of these provinces and that the existing biological communities there include a mixture of typically western, eastern and southern species. Typical western, Flindersian, species on Victorian reefs include: the algae Caulerpa brownii, Zonaria turneriana, Seirococcus axillaris, Carpoglossum confluens, Cystophora monilifera, Sargassum decipiens, Sargassum varians, Sonderopelta coriacea and Melanthalia obtusata; sea urchins including Holopneustes porossisimus and Holopneustes inflatus; the abalone Haliotis laevigata and Haliotis scalaris; and fish such as the horse-shoe leatherjacket Meuschenia hippocrepis, yellow-striped leatherjacket Meuschenia flavolineata and Victorian scalyfin Parma victoriae. Typical eastern, Peronian, species on Victorian reefs include: the algae Phyllospora comosa; the sea urchins Centrostephanus rodgersii and Holopneustes purpurascens; the sea squirt Pyura stolonifera; and fish including eastern hulafish Trachinops taeniolatus, silver sweep Scorpis lineolatus, black drummer Girella elevate, white Temperate reefs literature review 2 ear Parma microlepis and mado Atypichthys strigatus. Prominent southern, Maugean, species on Victorian reefs include: algae such as the string kelp Macrocystis angustifolia, bull kelp Durvillaea potatorum, Splachnidium rugosum, Zonaria angustata and Cystophora torulosa; the sea stars Patiriella brevispina, Nectria ocellata and Fromia polypora; and fish including southern hulafish Trachinops caudimaculatus and southern sea carp Aplodactylus arctidens. In addition to provincial influences, Victorian communities are also composed of species distributed throughout all of southern Australia including algae such as common kelp Ecklonia radiata, Pterocladia capillacea, Phacelocarpus peperocarpus, Haliptilon roseum, Sargassum sp. and Cystophora sp.; the common sea urchin Heliocidaris erythrogramma; and the eleven armed sea star Coscinasterias muricata. The high turnover of species in the central Victorian area was reaffirmed in a study based on examining echinoderm and crustacean species distributions along the coast of southern Australia by O'Hara (2000a). O'Hara (2000a) analysed museum collections sampled from within 62 spatial units between Western Australia and northern New South Wales. The study determined that 49% of the total species in southern Australia (739) occur in Victoria (362) and the species distributions and turnover were consistent with previous biogeographical classifications (Bennett and Pope 1952; Edgar 1984; Womersley and King 1990). Given the fact that these different regions meet in Victoria, allowing comparative work to be carried out at similar latitudes within a relatively small geographic area, surprisingly little work has been conducted on the ecology of rocky reefs in the State. Waters et al. (in press) recently provided an a priori test for the existence of Maugean, Flindersian and Peronian biogeographical provinces across southern Australia. The study quantitatively analysed distributional data of 1,500 algal species and identified the three distinct biogeographical assemblages, consistent with previous biogeographical classifications (Bennett and Pope 1952; Edgar 1984; Womersley and King 1990). The authors recommended that broad provinces be applied as a regional framework for understanding and managing Australia’s marine biodiversity, particularly for integrating the ongoing discovery of biological variation at finer scales. The authors support their recommendation through (1) arguing against the relevance of a biogeographical classification of Australia’s coastline based on physical variables (IMCRA 1998) and (2) documented evidence for ecological variation (aside from species composition) across southern Australia. The Interim Marine Coastal Regionalisation of Australia Technical Group, IMCRA (1998), used measurements of bathymetry, coastal geomorphology, sediments, currents, tides, water chemistry and water temperature to classify Victoria into six bioregions (Table 1). Waters et al. (in press) argue that the broad biological relevance of these bioregions remain unclear because they are derived from gross biophysical features rather than based on large numbers of taxa. The authors go on to question the effectiveness of IMCRA (1998) studies in defining regional biodiversity and shaping conservation policy. Evidence exists for ecological variation across southern Australia aside from species composition (Connell and Irving 2009). Connell and Irving (2008) sought to provide a biogeographical framework for the interpretation of variation in the ecology of, and threats to, subtidal rocky landscapes. The study quantified the frequency and size of patches of major benthic habitats across the southern coast of Australia to establish biogeographical patterns that may result from contrasting regional-scale processes. Key findings from the study include distinct biogeographical patterning in the extent of kelp forests as related to the Flindersian and Peronian provinces. Flindersian coasts have higher kelp forest cover and higher kelp forest heterogeneity relative to Peronian coasts. The patchwork of forests in the Peronian province is created by the foraging activities of the black urchin Centrostephanus rogersii, a species that is not recorded from the Flindersian province. Distinct differences in concentrations of nutrients, chlorophyll a, kelp forest canopy-morphology, kelp forest understorey associations and broad degree of wave exposure between Flindersian and Peronian coasts also support the finding of ecological variation, aside from species composition, across southern Australia (Connell 2007; Connell and Irving 2008; 2009; Waters et al. in press). Finer-scale bioregions in Victoria have also been described in order to better understand marine biodiversity at the ‘habitat’ and ‘community’ levels. Ferns et al. (2000) provided a review of classifying Victoria’s marine ecosystems, recognising the importance of both physical and biological factors and the use of qualitative and quantitative attributes. The authors acknowledge previous classifications at the broad-scale bioregion level, and then describe two approaches in assessing and understanding marine biological communities at the ‘habitat’ and ‘community’ levels. The first approach is to classify intertidal and subtidal marine ‘habitats’ using qualitative attributes collected from remote sensing and field survey techniques. Qualitative descriptions of seafloor substratum and dominant biota are generally arranged in a hierarchical system to create Marine Habitat Classes (MHCs). MHCs are applied over scales of 1 m – 100 km that have distinct physical and/or biological ‘habitat’ attributes. MHC attributes were identified for intertidal and subtidal areas by Ferns et al. (2000) (Tables 2 and 3). Edmunds et al. (2000) also provided a quantitative classification of the biological communities in Victoria. The classification was derived from quantitative sampling of biological communities of macrophytes, macroinvertebrates and fish associated with rocky reefs using visual census techniques. These biological data provided attributes for specifically defined Marine Ecological Communities (MECs), which were applied to areas at scales from 1 m – 1 km and consisted of distinct biological communities. The results of the study indicated that there was a considerable overlap of the provincial influences, particularly in the area of central Victoria where the Flindersian components extend as far east as Cape Liptrap, and the Peronian components extend as far west as Cape Otway. Ferns et al. (2000) state that MECs potentially serve as useful indicators for reporting on the long-term status of marine communities. Presently, MECs for macrophytes, invertebrates and fish have been delineated for a selection of subtidal reefs across four areas of coastline in Victoria including Port Phillip Heads, Phillip Island, Bunurong and Wilsons Promontory (Ferns et al. 2000). But due to the lack of similar Temperate reefs literature review 3 studies determining MECs along the remainder of the Victorian coast, local temperate reef community information is scarce. This is, however, gradually being addressed. In recent years, a number of studies have examined Victoria’s subtidal reefs using combinations of georeferenced aerial and satellite images, echo-sounders, GIS and ground-truthed video drops/transects and diver surveys. For example, the “Victorian Marine Temperate reefs literature review 4 Habitat Mapping Project” and other projects are covering reefs along the majority of Victoria’s coastline (Bax and Williams 2000; Bax and Williams 2001; Beaman et al. 2005; Ball and Blake 2007a; b; Holmes et al. 2007; Monk et al. 2008; Blake et al. 2009a; b; Rattray et al. 2009). These surveys have described a wide range of reefs along the Victorian coast at the habitat level and broadly describe the make-up and distribution of biota in relation to substrate type at the community level. Oceanographic and productivity context of Victorian reefs Central Victoria The oceanography of the central area of Victoria is dominated by the relatively shallow waters of Bass Strait. The Strait consists of a shallow platform, mostly about 70 m below sea level, flanked by 4-5 km deep ocean to the east and west and by land to the north and south. Although tidal currents can be strong in certain areas, such as the eastern and western entrances to Bass Strait (Black 1992), the net tidal circulation tends to be small. In central Victoria, the local tidal dynamics are strongly influenced by the two large bays, Port Phillip Bay and Western Port. The primary determinants of net water movement in the region are wind-driven currents and coastal trapped waves (Middleton and Black 1994). The density structure of Bass Strait ranges from well mixed in winter to strongly stratified in central areas in summer (Baines and Fandry 1983). Bass Strait is generally considered to have a low nutrient status because nutrient rich water from the deep ocean is generally absent or diluted (ASR 2008). Port Phillip Bay is a large (2000 km2), semienclosed, predominantly tidal embayment linked to the ocean of Bass Strait by a narrow entrance. The hydrodynamics are characterised by an entrance region where fast (3 m s-1) ebb and flood jets dominate the circulation, a large flood-tidal delta known as the Sands region, where strong currents occur in the major channels, and an ‘inner’ zone where tidal flows are weak and circulation is predominantly by wind-driven currents (Black et al. 1993). Tides are semidiurnal and the amplitude inside the bay is less than 1 m. Salinity in Port Phillip Bay is essentially marine and recently has become hypersaline in comparison with Bass Strait (Nicholson and Longmore 2009). Although there is significant nutrient input to the Bay from sewage treatment, effects of eutrophication are presently only seen in localised areas. Western Victoria The oceanography of western Victoria is influenced by the major western boundary current: the Leeuwin current. The winter circulation in Victoria is characterised by a west to east coastal current (Cirano and Middleton 2004). The eastward coastal current is partly driven by the Leeuwin current to the west (Cirano and Middleton 2004). The Leeuwin current is a warm water current, and the significant inter-annual variation that has been recorded in the eastern coastal current (Cirano and Middleton 2004) would be likely to lead to inter-annual variation in water temperature to the west of Bass Strait. The Leeuwin current, and by extension the eastern coastal current, is a warm, low-salinity current that is considered to be nutrient poor (Connell 2007; Suthers and Waite 2007). Eastern Victoria Eastern Victoria is influenced by the major eastern boundary current: the East Australian Current (EAC) (Suthers and Waite 2007; ASR 2008). The EAC often generates warm-core eddies, or circular flows, up to several hundred kilometres across. These eddies drift south and can be found off the central east coast of Tasmania (Suthers and Waite 2008; ASR 2008). Although most of the water from the EAC is directed into the Tasman Sea, some EAC water also enters eastern Victoria, especially over summer. The EAC is characterised by upwelling in a number of areas (Suthers and Waite 2007) and is generally considered more nutrient rich and productive than the Leeuwin current (Connell 2007). Upwelling As well as the influence of broad-scale currents and their associated nutrient regimes, local-scale areas of upwelling, with associated nutrient and productivity increases, can occur though-out Victoria when wind and oceanographic conditions are suitable. Upwelling is an oceanographic phenomenon where surface winds along coastal areas drive dense, cool, and nutrient-rich water towards the surface. Upwelling has been recorded in eastern Victoria (Gibbs et al. 1986; Rochford 1977; Butler et al. 2002b) and to a lesser extent in central areas of Bass Strait (ASR 2008). By far the strongest area Temperate reefs literature review 5 of upwelling and nutrient enhancement in Victoria occurs in the west. The Bonney upwelling is the most prominent upwelling in southeast Australia and is driven by the prevailing south-easterly winds in the region. Between November/December and March/April, upwelling plumes are regularly observed along the Bonney Coast from Robe, SA to Portland, Vic which make the area highly productive (Butler et al. 2002b), and all reefs in the area may be influenced to some degree by this phenomenon. The Bonney Coast is within the Otway region as classified by the IMCRA (Butler et al. 2002b) and was described by Womersley (1984) as being part of the Maugean province, of which it forms the western extension (Butler et al. 2002b). Temperate reefs literature review 6 Current monitoring Monitoring The Victorian Subtidal Reef Monitoring Program (SRMP) and Intertidal Reef Monitoring Program (IRMP) were established by the Victorian Government to provide information on the status of Victorian reef flora and fauna (focussing on macroalgae, macroinvertebrates and fish). The SRMP uses standardised underwater visual census methods based on an approach developed and applied in Tasmania by Edgar and Barrett (1997). This includes monitoring the nature and magnitude of trends in species abundances, species diversity and community structure through time, with particular emphasis on Marine National Parks and Sanctuaries in Victoria (Edmunds et al. 2003). The SRMP was initiated in May 1998 with 15 sites established on subtidal reef habitats in the vicinity of Port Phillip Heads Marine National Park. Since then, the SRMP has been expanded to reefs in the vicinity of the Bunurong Marine National Park (12 sites), Phillip Island (6 sites), and Wilsons Promontory Marine National Park (20 sites) and a further twelve Marine National Parks and Marine Sanctuaries including: Point Cooke, Jawbone, Ricketts Point, Merri, Marengo Reef, Eagle Rock, Beware Reef and The Arches Marine Sanctuaries and Point Addis, Cape Howe, Point Hicks and Twelve Apostles Marine National Parks. This program is currently implemented by Parks Victoria, in association with the Department of Sustainability and Environment (Edmunds et al. 2006a). The data include quantitative estimates of large mobile fishes, cryptic fishes, megafaunal invertebrates and macroalgal species. The IRMP was initiated in April 2003 with 14 sites established on intertidal reef habitats within, and in the vicinity of, the following marine protected areas: Point Addis Marine National Park and Point Danger, Barwon Heads, Point Cooke, Jawbone, Ricketts Point and Mushroom Reef Marine Sanctuaries. Although the monitoring program will begin to adequately reflect average trends and patterns as the surveys continue over longer periods such as multiple years to decades (Edmunds et al. 2003), Ball et al. (2006) state that macrophyte communities identified in the study have been generally compatible with dominant reef biota MHC categories. The Abalone Assessment Monitoring program run by Department of Primary Industries (DPI) is an annual fishery independent survey across 250 subtidal reef sites in Victoria that commenced in 2002. The data are collected by underwater visual census and includes quantitative estimates of abundance of abalone and prevalent organisms other than abalone such as macroalgae, predators and competitors and abiotic features. Marine Habitat Classification Since the Victorian interim marine habitat classification scheme presented in Ferns and Hough (2000) there has been several steps forward in improving marine habitat classification not only on a temperate reef level but on a nationwide marine habitat level. These advancements will allow future studies to further our understanding of the distribution and abundance of different temperate reef habitats in Victoria. Ball et al. (2006) reviewed and documented the relationship between relevant Australian and international local-level marine habitat classification systems and the Victorian interim marine habitat classification scheme presented in Ferns and Hough (2000). The authors then presented a revised marine habitat classification system to support mapping of shallow (assumed 0 - 20 m depth) habitats at Victoria’s Marine National Parks and Sanctuaries. The primary habitat classification scheme divides classification into five levels of modifiers to classify habitats mapped from underwater (Table 3). The purpose of this habitat mapping classification is to assist in the selection and evaluation of candidate representative marine protected area locations, to allow more accurate description of the spatial extent and distribution of shallow seabed habitats, and to provide more detailed biological information on marine protected areas to support assessment of management performance. Temperate reefs literature review 7 Ecosystem services Ecosystem services are the direct and indirect benefits supplied to human societies by natural ecosystems (Daily et al. 1997) including, for example, key fishery species in the reef community, or productivity values associated with the reef. Temperate reefs are known for their high biological complexity, species diversity, species richness, level of endemism and productivity (Keough and Butler 1996; Edmunds et al. 2003). Temperate reef habitats have social and cultural values including indigenous, aesthetic, recreational and historical aspects (Keough et al. 1990; Edmunds et al. 2006a). They also hold significant economic value by supporting two of the most valuable commercial fisheries in Australia, in abalone and rock lobster, plus other valuable activities such as recreational fishing, diving and other tourism activities (Environment Conservation Council 2000; Edmunds et al. 2003; Department of Primary Industries 2009b). Keough and Butler (1996) add that the temperate reefs in Australia are important for commercial and recreational fisheries and for recreational diving, and they are potential sources of natural products for the pharmaceutical industry The subtidal and deep reefs (including the canyon) throughout Port Phillip Bay are described as ecological assets of Port Phillip Bay Temperate reefs literature review 8 (Port of Melbourne Corporation 2007a; b; Connell and Irving 2009). The south of the Bay, together with the Entrance, is popular for recreational diving because the water is generally clear and the area contains high quality diving sites associated with the deep canyon in the Entrance, marine national parks and shipwrecks. Key dive sites across the south of the Bay include Lonsdale Wall, which extends to depths of 45 m, and Popes Eye, which is a renowned location for trainee divers, snorkellers and for bird watching and general sightseeing (Port of Melbourne Corporation 2007a). An example of the importance of reefs in Port Phillip Bay in terms of ecosystem services is the recent State Government initiative to create artificial reefs for recreational angling in the Bay. This indicates that natural reefs are considered to be an important and limited resource (P. Hamer, pers comm.) Other examples of the ecosystem services provided by reefs in Port Phillip Bay and elsewhere include protection from beach erosion by acting as a wave break, and also associated algae act as a nutrient sink and site of detritus production that underpins the detrital food chain in soft bottom habitats. Physical habitats and biological communities Intertidal reefs Intertidal reefs vary in structure from steep sloping rock faces to relatively flat or gently sloping boulder fields and rock platforms, with a variety of features including cobble fields, vertical steps, undulations in the reef, crevices, patches of sand and rock pools caused by a variety of processes such as weathering (Edmunds et al. 2004). Bennett and Pope (1952) surveyed 16 sites on exposed intertidal reefs along the coast of Victoria and in spite of differences in localities, five major zones and subsequent community assemblages were identified: Melaraphe-lichen zone: species present include herbivorous molluscs (Nodilittorina spp., previously called Melaraphe spp.), lichens, crustaceans such as small crabs, amphipods and isopods, and occasionally predatory gastropods. Barnacle-mussel zone: species present include barnacles and mussels. The mixed algal turf or Galeolari zone: species present include mixed algae in exposed areas with the serpulid Galeolaria caespitosa in sheltered areas. The Poneroplax or “Bare” zone: species vary along the coast and with wave action. Chitons of the genus Poneroplax are always present, and on many shores east of Cape Otway ascidians are found. The large brown algae zone: species present include large fucoid kelp algae. Such patterns of vertical distributions in organisms are well chronicled on rocky shores internationally, for example bare zones at the mid shore level (Mettam 1994; Kaehler and Williams 1997). Zonation of algae in Port Phillip Bay was briefly reviewed by Spencer (1970), and has been examined on basalt reefs (King et al. 1971; O'Brien 1975; Brown et al. 1980). Clear vertical zones were apparent on reefs in the Bay and while species present varied to some extent between sites, some of them were commonly found. For example: Upper eulittoral zone: species include Enteromorpha spp. Mid eulittoral zone: species include Porphyra spp, Ulva spp, Gelidium pusillum Lower eulittoral zone: species include Codium sp., Caulerpa sp., Polysiphonia sp. More recent studies (O'Hara et al. in press) have collected data on 65 intertidal reefs across Victoria as part of an effort to detect impacts on assemblages, and there are ongoing qualitative surveys being conducted at Barwon Heads by local school children. Several recent monitoring studies of Victoria’s Marine Protected Areas have described the dominant biota present on Victorian temperate reefs. One of the most common algal species on intertidal reefs is the perennial fucoid alga Neptune’s necklace, Hormosira banksii, which can form extensive monotypic stands at midtidal levels of rock platforms (Keough and Quinn 1998). Other common algae species that can form mats with or without the presence of H. banksii include the green algae Ulva spp. and Enteromorpha spp, coralline algae and filamentous brown algal turfs (Edmunds et al. 2004). Less conspicuous is a thin layer of microscopic algae growing directly on the surface of the reef, which is an important food source for species of grazing molluscs (Edmunds et al. 2004; Gilmour and Edmunds 2007; Stewart et al. 2007). The invertebrate fauna on intertidal reefs, which tend to exhibit patterns of zonation in the same way as shown by algae (King et al. 1971), are dominated by herbivorous and predatory molluscs. Common herbivorous species on Victoria’s shores include the top shell Austrocochlea porcata, the variegated limpet Cellana tramoserica and conniwinks Bembicium spp. Less common species include the warrener Turbo undulatus, the black nerite Nerita atramentosa and the predatory Temperate reefs literature review 9 gastropods Cominella lineolata and Lepsiella vinosa (Edmunds et al. 2004; Gilmour and Edmunds 2007). Sessile invertebrate species create encrusting mats on the surface of the reef such as the mussels Xenostrobus pulex and Brachidontes rostratus and tubeworms Galeolaria caespitose, whilst there are a variety of other mobile invertebrates such as small crabs and fish species that are either present in the intertidal zone throughout the tidal cycle and refuge in rockpools, or move into the area at high tide (Edmunds et al. 2004; Gilmour and Edmunds 2007; Stewart et al. 2007). As is common on shores elsewhere, both in Australia and worldwide (Little et al. 2009), differences exist between biota present on exposed intertidal reefs along the Victorian coastline and sheltered intertidal reefs within Port Phillip Bay. Gilmour and Edmunds (2007) surveyed intertidal reef biota of Central Victoria’s Marine Protected Areas and found species richness of algae and invertebrates, and algal cover, to be higher on the exposed intertidal reefs along the coastline than on the sheltered intertidal reefs inside Port Phillip Bay. Stewart et al. (2007) reaffirmed their findings and also found that mat forming mussels were components of more intertidal reef communities outside Port Phillip Bay than inside the Bay. Intertidal reefs in Victoria are known to show the same general patterns as those in eastern Australia (Bennett and Pope 1952). Underwood and Kennelly (1990) stated that “kelps are represented by Durvillaea potatorum, Ecklonia radiata and Phyllospora comosa; Macrocystis angustifolium is present in some places. Cystophora intermedia are found in eastern Victoria and reappear in South Australia. Tunicates, such as the cunjevoi Pyura stolonifera, are only found in some places in Victoria and low-shore regions are occupied by large chitons and encrusting calcareous algae. Above the regions dominated by algae are areas occupied by barnacles. Conspicuous amongst these barnacles are bands of foliose algae (e.g. Splachnidium rugosum and Ileafascia in summer).” Temperate reefs literature review 10 Several authors have investigated specific groups of species in Victoria. For example, a review by Sanderson (1997) described algal communities in more depth and Light (1992) reviewed literature on the benthic flora of Port Phillip Bay, which included information from both soft and hard substrates. Surveys of Port Phillip Bay in the 1950-60s described 173 species of subtidal algae, which included numerous species attached to hard reefs, particularly in the Port Phillip Heads region (Womersley 1966). Womersley (1966) noted that species richness was lower inside than outside the Bay and suggested that this may be due to a variety of factors including differences in water depth and temperature. He also mentioned the comparable lack of rocky reefs within Port Phillip Bay. Hope Black (1971) used this data to examine distributions with substrate, in particular concentrating on the importance of reefs in Port Phillip Bay as habitat and summarised the distribution of reefs in Port Phillip Bay as 4 main groups in respect to rock type: 1. Dune limestone: in and adjacent to Port Phillip Heads, including Popes Eye (artificial basalt structure) 2. Oligocene basalt: from Corio Bay to Williamstown 3. Tertiary ironstone of the Miocene clays and sandstones: north and east shores 4. Granites: extending from Martha Pont seawards. Form off-shore reefs. Light and Woelkerling (1992) summarised descriptions previously made of algae in the intertidal and subtidal zones of Corio Bay (Womersley 1966; Hope Black 1971), the Werribee region (Womersley 1966; Spencer 1970; Hope Black 1971; Brown et al. 1980), Altona Bay – Hobsons Bay (Spencer 1970), Carrum and Portsea, and discussed unpublished data for Werribee (Brown et al. 1980), Gloucester Reserve (O'Brien 1975), Williamstown and Portarlington (Lewis 1975). Subtidal reefs Subtidal reefs are defined here as reef that is never exposed to the air from tidal influences and generally covering depths of 2.5-20 metres. They can be split into two categories: 1. Exposed subtidal reefs 2. Sheltered subtidal reefs Exposed subtidal reefs are physically characterised by a high degree of exposure to wave and current energy. Exposed subtidal reefs exist along the majority of the Victorian coastline and include the reefs at the entrance of Port Phillip Bay, due to their high degree of exposure to ocean swells and tidal energy (McShane et al. 1986; Port of Melbourne Corporation 2007a). Certain taxa have been described in some detail off the coast of Victoria yet gaps still remain in our knowledge. For example, a review of opisthobranch molluscs (Burn 2006) in Victoria described the range of species that occur in habitats such as the rocky intertidal zone and subtidal rocky reefs from Cape Otway to Wilsons Promontory, but noted that little is known of species in shallow waters off eastern Victoria (Wilsons Promontory to Cape Howe). Algae In Victoria, subtidal reef communities are typified by their prominent biota of algae (Keough and Butler 1996; Edmunds et al. 2006a; Connell 2007). Sanderson (1997) described how reefs show broad patterns of zonation along the depth gradient: Hormosira banksii is the dominant species observed on intertidal rock platforms; Durvillaea potatorum with mixed brown and green algae inhabits the seaward edge of intertidal rock platforms; Phyllospora comosa occurs in large beds at depths of ~3–5 m; at exposed sites, Ecklonia radiata is typically observed at depths >7 m and often growing with P. comosa; and giant kelp Macrocystis angustifolia occurs at Port Phillip Heads MNP - Point Lonsdale and at Point Nepean. Other research on subtidal algae on exposed subtidal reefs in Victoria is thoroughly reviewed by Light and Woelkerling (1992) and Sanderson (1997). These also include reports on subtidal reef algae at Black Rock between Ocean Grove and Torquay (Rollings et al. 1993; Chidgey and Marshall 1994) and other areas throughout Victoria and the region (Cheshire and Hallum 1989; Land Conservation Council 1993). On exposed subtidal reefs at the entrance to Port Phillip Bay, high vertically-structured brown algae (kelps) such as Macrocystis angustifolia, Ecklonia radiata and Phyllospora comosa are often dominant. The green alga Caulerpa is also usually present plus a wide variety of low structured, understorey red algae species including Plocamium, Pterocladia, Melanthalia and Coralina (Port of Melbourne Corporation 2007b). Vegetation has been surveyed at a number of subtidal reef sites along the coast of Victoria resulting in the identification of two distinct vegetation groups together with the following observations recorded by O'Hara (2000b; 2001): Ecklonia/Phyllospora: Form dominant canopies in many exposed open-coast localities across Victoria. Phyllospora comosa forms a dense canopy on high relief, shallow (3 to 8 m), exposed or semi-exposed reefs but is often absent from low-relief reef. Ecklonia radiata often occurs in combination with Phyllospora or in combination with Macrocystis, Cystophora and other brown algae when Phyllospora is absent and is the dominant species on deeper areas of reef (Ball et al. In prep). Cystophora/Sargassum: A diverse mixed-algal assemblage occurs in some Victorian subtidal reefs where the usual Phyllospora/Ecklonia canopy is absent. These sites are visually characterised by the fucoid algae Cystophora, Sargassum, Seirococcus and Acrocarpia, with many other brown, red and green algae interspersed (including Ecklonia and Macrocystis). Invertebrates Exposed subtidal reefs in Victoria typically have high abundances of large-mobile predatory and grazing invertebrates (Edmunds et al. 2006a). Predatory mobile invertebrates include: rock lobster Jasus edwardsii, red bait crab Plagusia chabrus, dogwhelk Dicanthais orbita, octopus Octopus maorum and seastars Coscinasterias muricata. Grazing mobile invertebrates include: abalone Haliotis rubra and H. laevigata, warrener Turbo undulatus and sea urchins Centrostephanus rogersii, Heliocidaris erythrogramma, Holopneustes and Amblypneustes (Edmunds et al. 2000; Australian Government 2005; Port of Melbourne Corporation 2007b; c). The low abundance of the grazing urchin Centrostephanus rodgersii on exposed reefs Temperate reefs literature review 11 throughout the majority of Victoria results in urchin barren habitat being restricted to the fareast where it is present in high abundance (Keough and Butler 1996; O'Hara 2000b; Ferns 2003; Department of the Environment and Heritage 2005). Several studies have been conducted on the creation and maintenance of urchin barrens elsewhere in southern Australia and New Zealand (Andrew and MacDiarmid 1991; Andrew 1993; Andrew and O'Neill 2000). In Victoria, urchin barrens have been described at Cape Howe Marine National Park (Ball and Blake 2007b). There are also various small species of crustaceans and molluscs that occupy niches as grazers, predators or foragers (Edmunds et al. 2006a). Sessile invertebrates present on exposed subtidal reefs can be found in several body forms: Prominent colonial or modular sessile fauna such as sponges, ascidians, soft corals, hydroids and bryozoans are abundant on subtidal reefs, usually in high abundances within well-shaded rock faces, overhangs and caves, and on the underside of boulders (Keough and Butler 1996; Keough 1999) Solitary or unitary forms of sessile fauna include barnacles, some ascidians (eg. Pyura), bivalve molluscs and polychaetes (Keough and Butler 1996). Fish Fish are a dominant biotic component of exposed subtidal reef ecosystems in Victoria with wrasse (Labridae), morwong (Cheilodactylidae) and leatherjacket (Monacanthidae) making up the majority of species (Edmunds et al. 2000; Edmunds et al. 2006a). Wrasse are a roaming predatory species that feed on small molluscs and crustaceans, while morwongs are herbivorous or omnivorous and leather jackets are generally omnivorous or carnivorous picker-type species (Edmunds et al. 2000). Exposed subtidal reef fish species also commonly include herring cale Odax cyanomelas, sea sweep and scaly fin (Port of Melbourne Corporation 2007c). Reef fish tend to include algavores, carnivores and omnivorous species. Russell (1983) noted that “most rocky reef fishes have broadly generalised feeding habits, and foods taken mainly reflect those organisms of suitable size that are abundant and most readily available”. Gillanders (1995) has shown that the diet of rocky reef fish of different size classes can vary Temperate reefs literature review 12 with depth and habitat, with juveniles feeding in shallow areas of fringing habitat and adults in deeper turf and barrens habitats, reflecting the distribution of the species with depth. Some reef-related fish species show regular patterns of seasonal movement of adults on and off reefs, such as Hypoplectrodes maccullochi at Cape Banks NSW, possibly due to changes in prey abundance or because adults move to other areas to spawn (Webb and Kingsford 1992; Holbrook et al. 1994) Sheltered subtidal reefs are physically characterised by a low degree of exposure to wave and current energy. Sheltered subtidal reefs occupy a relatively small part of Port Phillip Bay, about 8 km2, or less than 0.5%, of the seafloor (McShane et al. 1986; Port of Melbourne Corporation 2007c). These reefs are generally within 1 km of shore in shallow water depths less than 4 m (Port of Melbourne Corporation 2007c). Port of Melbourne Corporation (2007c) described the distribution and lithology of Port Phillip Bay reefs. Basalt reefs exist along the northern shoreline at Williamstown extending west to Point Lillias and sandstone reefs exist along the north-eastern shoreline from Ricketts Point to Point Ormond. There are also patches of reefs on the south-eastern shoreline from Dromana to Frankston and on the Bellarine Peninsula from Clifton Springs to St Leonards (Port of Melbourne Corporation 2007a). Several artificial reefs exist within Port Phillip Bay, including newly deployed reefs for recreational fishing in the north of the Bay. Previous studies such that carried out by Lewis (1983) have noted that algae on artificial structures in Port Phillip Bay usually includes several introduced species. Algae Research on algae in Port Phillip Bay is reviewed in Light and Woelkerling (1992) and Sanderson (1997), and includes reports on subtidal reef algae in: the Werribee region (Brown et al. 1980); Williamstown, St Leonards, Corio Bay (King et al. 1971; Lewis 1975); Altona and Portarlington (Spencer 1972); and Hobsons Bay (O'Brien 1975). The surfaces of sheltered subtidal reefs in Port Phillip Bay are typically patchier near the exposed entrance, with various red and brown algae and the green algae species Caulerpa and Ulva, while other areas are characterised by the kelp Ecklonia radiata or the introduced species Undaria pinnatifida (Campbell 1999; Port of Melbourne Corporation 2007c). macroalgal cover, and possibly the effects of spear fishing (Jenkins et al. 1996) Invertebrates Research on invertebrates on subtidal reefs within Port Phillip Bay is sparse, although some research is available that has examined relationships between invertebrates in the bay, such as studies by Day et al. (1995) and McShane and Smith (1986) on starfish predation. Fish often display complex relationships with their surroundings. A whole suite of factors are known to affect temperate reef fish assemblage structure and populations. For example, habitat type and movement patterns can influence the size range and age classes found at different sites (Wheatley 2000). Fish Sheltered reef fish species commonly include southern hulafish Trachinops caudimaculatus, weed fish Heteroclinus perpicillatus, old wives Enoplosus armatus, toadfish (Aracanidae), leatherjackets (Monacanthidae), globe fish (Diodontidae), zebra fish Girella zebra, blennies (Blennidae) and seahorses Hippocampus sp.. Wheatley (2000) examined fish assemblage structure, species richness and density on sheltered reefs in Port Phillip Bay in relation to macro-algae, depth, substrate type etc., as well as how habitat mediates recruitment, competition and predation, with an emphasis on the leatherjackets Meiischenia freycineti and Meiischenia hippocrepis. This study found that fish assemblages were most similar on reefs that were closer together than distant reefs, and suggested that these patterns were due to similarities in larval supply, wave action and tidal movement as well as to differences in macroalgal assemblage structure. M. hippocrepis were only recorded on reefs, while M. freycineti were mainly found on reefs as adults following recruitment to seagrass beds. Mean growth rates differed between adults of the two species on different reefs, and adults appeared to be permanent residents on individual reefs, with no evidence of movement between reefs being found. While M. freycineti juveniles and subadults moved between coastal seagrass beds and offshore reefs over sand patches, the same sand patches appeared to restrict movement of adults, a pattern of movement also described for this and other Monacanthid species in NSW (Bell et al. 1978). Coleman (1972) collected fish from sheltered rocky reefs at Williamstown, Sandringham, Rickett’s Point, Mornington, Mt. Martha, Safety Beach and Sorrento Bay. Fish from 31 families were recorded and observations of basic ecology and biology were made including habitat use and feeding behaviour. This study briefly discussed how food availability, substrate type and exposure may influence the distribution of these species. Sheltered subtidal reefs are also important to snapper Pagrus auratus and King George whiting Sillaginodes punctata at different stages of their life cycles (Port of Melbourne Corporation 2007c). The structure of fish communities inhabiting reef-algal and unvegetated sandy areas at six sites has been studied by underwater visual census over 18 months in Port Phillip Bay (Jenkins et al. 1996). A total of 75 species representing 36 families were recorded. Species richness and total abundance varied both spatially and temporally but were always greater on the reefs (Jenkins et al. 1996). In general, the northern bay sites were characterised by small cryptic species with an increase in conspicuous water column species in the south of the Bay (Jenkins et al. 1996). Differences in community structure between these sites may be related to reef topography, Such patterns of ontogenetic movement between habitats as individuals grow has been reported for other species in the Bay such as Sillaginodes punctatus (that moves between seagrass beds/reef-algae and sand areas ) (Jenkins et al. 1996; Jenkins and Wheatley 1998), as well as elsewhere in the region, e.g., Pseudolabrus celidotus (Jones 1984) in New Zealand and Achoerodus viridis (Gillanders 1997b; Gillanders 1997a) in NSW. Temperate reefs literature review 13 Deep reefs Deep reef habitat is defined here as rocky habitat at depths greater than 20 m (Ball and Blake 2007a; Port of Melbourne Corporation 2007b). In western Victoria, recognised deep reef sites include Discovery Bay Deep, offshore Cape Bridgewater and Cape Nelson, Port Fairy Deep, offshore Moonlight Head, Cape Otway, Apollo Bay and Nine Mile Reef (Edmunds et al. 2006b; Ball and Blake 2007a; Ball et al. in prep). Other features include a submarine scarp between Barwon Heads and Cape Otway on the Otway Shelf (Jennings 1958) and a cliff at ~45 m depth that extends for ~20 km between Point Roadnight and Sugarloaf Creek (Gill et al. 1980). In central Victoria, deep reef sites include Port Phillip Heads, including The Canyon, Point Addis and Wilson’s Promontory (Edmunds et al. 2006b; Ball and Blake 2007a). Deep reefs habitats are also located in Port Phillip Bay itself at Schnapper Deep, Portsea Hole, Spectacular Reef and Far Side Reef, and The Rip (Edmunds et al. 2006b; Port of Melbourne Corporation 2007a). In eastern Victoria, smaller areas of deep reef exist near Point Hicks and Cape Howe (Edmunds et al. 2006b). Mapping has been performed using video transects and side-scan sonar to provide quantitative descriptions of reef biota on sites including: granite reefs such as “Point Hicks Reef”and“New Zealand Star Bank”; low relief sandstone/limestone reefs such as “Broken Reef”; and high/low relief limestone reefs such as the ”Howe/Gabo complex” (Bax and Williams 2001). Broader-scale surveys have also been conducted of substrate type and benthic organisms throughout the Bass Strait which have included reefs such as “New Zealand Star Bank” (O'Hara 2002; Passlow et al. 2006). At New Zealand Bank, Beaman et al. (2005) described the biota that occurred on broadly categorised habitats. In this particular study, “high relief granite” outcrops at ~30-45 m were described as closely resembling those of similar habitats along the coast of NSW, where communities include large sponges, ascidians, cnidarians, bryozoans and reduced algal cover (Underwood et al. 1991; Andrew 1999; Beaman et al. 2005). “Deep reef/urchin barrens” described at this site at ~40-50 m (Beaman et al. 2005) closely resemble those described in NSW and elsewhere (Underwood et al. 1991), with large concentrated patches of the urchin Centrostephanus rodgersii on deep reef, inhabiting areas consisting Temperate reefs literature review 14 predominantly of encrusting algae and, where urchins are low in number, many sessile invertebrates such as sponges and seawhips. Edmunds et al. (2006b) states that few studies have been done on deep reef biology due to the logistical difficulties associated with working at these depths. While some deep reef invertebrate species have been identified, most remain unidentified due to the paucity of collected specimens. Consequently, our ecological understanding of deep reef assemblages is limited, especially with respect to patterns and factors that influence the diversity and abundance of deep reef species. In general, worldwide research on deep reefs remains sparse in comparison with that on shallow reefs. Invertebrates Deep reef biota is typified by invertebrate animals rather than algae, usually in the form of sessile, filter feeding fauna (Tissot et al. 2006; Sanchez et al. 2009). Organisms such as sponges, octocorals, bryozoans and ascidians usually dominate rock faces on deep reefs (Keough and Butler 1996; O'Hara et al. 1999; Edmunds et al. 2006b). For example, the rocky wall within Portsea Hole is characterised by an abundance of sponges, ascidians and bryozoans (Elias et al. 2004; Edmunds et al. 2006b). This is partly due to the ability of species such as sponges to survive in low light conditions that algae is unable to survive in (Sorokin et al. 2008). Communities of sessile invertebrate animals are sometimes collectively known as a ‘sponge bed’ (Butler et al. 2002a). Sponge bed species can be encrusting or grow in a variety of forms whilst attached to the substratum (Edmunds et al. 2006b). The most common algae present on deep reefs are encrusting coralline red algae which is able to tolerate low levels of penetrating light (Edmunds et al. 2006b). Sponge bed communities are the typical deep reef biota at sites across Victoria but there are differences in assemblages between deep reef sites (Edmunds et al. 2006b; Ball and Blake 2007a; Ball et al. in prep). Butler et al. (2002a) have surveyed sponges throughout the Bass Strait. Recent surveys have concentrated on the area of reef known as The Rip, in Port Phillip Heads, which is more complex than other reefs within the Bay and includes “a variety of substrate structures including vertical walls, caves, ledges, reef slopes, reef flats and steep-sided chasms” (Edmunds et al. 2006b). These diverse microhabitat types may result in a high diversity of species and (Edmunds et al. 2006b). Edmunds et al. (2006b) found assemblages of benthic fauna were different between all deep reefs within Central Victoria and Port Phillip Bay collectively. The Port Phillip Bay deep reef assemblages were dominated by sponges, occupying 70 to 90% of the rocky substratum. The Point Addis assemblage was dominated by upright sponges (arborescent, massive and flabellate growth forms), but cnidarians including hydroids were entirely absent. Wilson’s Promontory had a low coverage of encrusting sponges and hydroids, with high abundances of red and brown algae and the gorgonian fan Pteronisis sp.. The Port Phillip Heads assemblage was dominated by encrusting sponges, hydroids, ascidians and bryozoans, many of which are considered rare, and, according to Edmunds et al. (2006c) there are many identifiably different assemblage types present. Within Port Phillip Heads, further differences in assemblage structure were apparent between areas (Edmunds et al. 2006b): Catacombs Ridge, the Plateau and Nepean Bank contained a high abundance of encrusting sponges and hydroids Deep cobble habitats, such as Uelmans Deep were characterised by low abundances of sessile organisms Reef walls at ~25 m were dominated by encrusting osculated sponges and other sessile species, e.g. Portsea Hole Reef slopes in the area were characterised by dominant assemblages of “massive ruffled sponges, rambling grey encrusting sponge and other encrusting sponges”. Other sessile species that occur in the reef assemblages of Port Phillip Heads include hydroid fans, soft corals, gorgonian corals, crustose and arborescent bryozoans, colonial ascidians, and solitary ascidians (Edmunds et al. 2006b). It has been reported that over 271 validated species of sponges have been collected from Port Phillip Heads, which is a large proportion of the 523 species identified to date from Victoria, and the 1416 identified to date throughout Australia (Flora and fauna guarantee - scientific advisory committee 2009). Notably, 115 of the species recorded in Port Phillip Heads are unknown from any other area (Flora and fauna guarantee - scientific advisory committee 2009). However, detailed taxonomic studies are still incomplete on these species in Port Phillip Heads and as sponges are known to exhibit variable growth patterns in relation to currents, wave action, predation, etc. (Palumbi 1986; Kaandorp 1999; Bell and Barnes 2000; Hill and Hill 2002), and species can adapt their gross morphology to changes in their environment within weeks (Palumbi 1984; Kaandorp and Kluijver 1992), more in depth taxonomic studies are required to confirm these records. Similarly, this area also exhibits a high diversity of Bryozoans compared to other areas (Ponder et al. 2002), has been described as an area containing highly species rich hydroid fauna (Watson 1982), and contains at least one ascidian species known only from Port Phillip Heads (Flora and fauna guarantee - scientific advisory committee 2009). While studies of organisms on deep reefs in Port Phillip Heads remain sparse, work has been conducted elsewhere on species such as sponges that are predominant on this type of reef (Roberts and Davis 1996; Roberts 1996). While recent surveys of Port Phillip Heads have begun to identify those species that make up sessile communities, little is known about the mobile invertebrates that occur on deep reefs in Port Phillip Bay. This is partly due to sampling strategies that have been used, with video transects unable to record species that are often cryptic in nature and shelter in cracks and crevices in the substrate as well as within cryptic microhabitats created by sessile organisms. In addition, technical difficulties involved in surveying deep reefs also make this problematic (Edmunds et al. 2006b). Edmunds et al. (2006b) suggests that these organisms play an ecologically significant role, providing food for carnivorous fishes on deep reefs in Port Phillip Bay, and are likely to include a variety of crustaceans and molluscs. Fish Deep reef fish fauna include common subtidal reef fish species, but other species that are rare on shallow reef may also be present including boarfishes Pentacerotidae), splendid perch Callanthias australis, butterfly perch Caesioperca Lepidoptera and banded seaperch Hypoplectrodes nigroruber (Edmunds et al. 2006b). Fish assemblages typically begin to change at depths greater than 20 m, with the loss of the kelp- Temperate reefs literature review 15 associated wrasses and leatherjackets, and the appearance of deeper water species (O'Hara et al. 1999). On deep reefs within Port Phillip Bay, fish assemblages are reasonably diverse and include a variety of planktivores, roaming carnivores and omnivore/herbivores that occur in moderately high abundances (Edmunds et al. 2006b). The relationships between fish distributions and reef habitat in Victoria have not been examined to a large extent, apart from recent studies on reef down to ~60 m at Cape Howe Marine National Park (Moore 2008; Moore et al. 2009) using stereo-Baited Remote Underwater Video Systems (stereo-BRUVS). This study used habitat suitability modelling to successfully link the distribution of fish species with environmental characteristics including depth, substrate type and reef complexity (Moore et al. 2009). This allowed an examination of subtle differences in biological and topographic complexity and their importance in structuring species distributions. For example: The eastern blue grouper Achoerodus viridis, a carnivore which feeds on a variety of prey, was distributed in relation to the presence of solid reef and boulders The green moray Gymnothorax prasinus was linked positively to reef and broad-scale topographic complexity The eastern blue-spotted flathead Platycephalus caeruleopunctatus, was closely affiliated with sand (Hutchins and Swainston 2002) and was present in depths Temperate reefs literature review 16 of > 60 m over sediment areas and some reef-and-sediment areas, and in relation to algae and invertebrates The draughtboard shark Cephaloscyllium laticeps was present across all substrata in depths of ~ 60 m, and was predicted to be present out to the deepest regions of the park at ~110 m Surveys of habitat structure and fish community structure on deep reefs from 25-200 m from Gippsland Lakes – Cape Howe and southern NSW found a correlation between communities and depth, latitude and seabed type (Williams and Bax 2001). For example, 61% of fish species surveyed were associated with either reef or soft substrates, and the remainder were associated with both. Species that were associated only with reefs in the study included: butterfly perch Caesioperca lepidoptera, eastern orange perch Lepidoperca pulchella, bearded rock cod Pseudophycis barbata, chinaman leatherjacket Nelusetta ayraudi, barber perch Caesioperca razor, splendid perch Callanthias australis, striped trumpeter Latris lineate, mado Atypichthys strigatus, bastard trumpeter Latridopsis forsteri, common bullseye Pempheris multiradiata, maori wrasse Ophthalmolepis lineolata, longfin pike Dinolestes lewini, largetooth beardie Lotella rhacinus, bluethroat wrasse Notolabrus tetricus, southern conger Conger verreauxi, sergeant baker Aulopus purpurissatus, swallowtail Centroberyx lineatus, pigfish Bodianus sp., silver sweep Scorpis lineolata, and thresher shark Alopias vulpinus. Canyon reefs Assemblages within canyons vary with substrate depth, size, and complexity (Harris 2007; Williams et al. 2009b) and these habitats may be particularly sensitive to anthropogenic disturbance in the form of commercial fishing which is concentrated at ~50-1300 m depth (Larcombe et al. 2002; Williams et al. 2009b). anemones and ascidians (Port of Melbourne Corporation 2007d). Sponges make up 67% of the biological groups in the canyon, hydroids 14% and all other groups less than 2% each (Port of Melbourne Corporation 2007d). Edmunds et al. (2006b) also found encrusting sponges and hydroids to dominate these assemblages. Port Phillip Heads Other canyons The entrance of Port Phillip Bay contains a key geomorphological feature known as “The Canyon” which meanders more than 3 km from 1 km south‐west of Point Nepean through the Entrance and 1 km north‐west of Point Nepean inside the Entrance. It covers an area of ~100-120 ha, with a depth range to ~100 m, but also includes two shallow banks, Nepean Bank and Rip Bank, which lie in the shipping channel through the Entrance (Port of Melbourne Corporation 2007d). The canyon contains a distinctive ecological environment unlike other environments in the Bay or elsewhere in Victorian waters. Reef communities within the Canyon are present on mixed substrates of Aeolian calcarenite and basaltic rock and experience large volumes of water movement, with the tidal flow between the heads reaching speeds of up to 5 ms-1 (Edmunds et al. 2006b). The water passing through Port Phillip Heads carries high volumes of suspended particles such as sand on the flood tide and sediments from the Bay on the ebbing tide which reduce light levels, but increase nutrient levels (Edmunds et al. 2006b). Little is known about abyssal reefs found deep on the continental slope (>200-400 m), such as the “Cascade” off east Gippsland, which is thought to be dominated by cold water corals that form a habitat for a variety of invertebrate species (O'Hara et al. 1999). Invertebrate communities are common deep reef biota, but are difficult to study because of the strong tidal currents, ocean waves and seabed depths that are beyond the normal range of depth for scuba divers. Consequently, the Canyon’s habitats and associated biota have only been examined in detail by using remotely operated vehicles (ROVs) and, to a lesser extent, divers using underwater video and still photography (Port of Melbourne Corporation 2007d). These ecological studies confirmed that much of the rocky seabed beyond 20 m in the canyon consists of highly sculptured calcarenite reef (including vertical walls, pinnacles, crevices etc) with sandy and rubble patches on the flatter regions and in the deep basins. The permanent rocky habitat is totally covered with a range of sessile invertebrates, particularly encrusting sponge communities, with a variety of component biota including hydroids, bryozoans, Recent mapping of geomorphic features and assemblages from 200-2000 m has, however, been conducted in south-east Australia (Harris et al. 2005) and has been used in the design of deep water Marine Protected Areas (Harris 2007), although mapping is still incomplete at the current time (Williams et al. 2009b). As such, relatively little is known about organisms that inhabit deep reefs and rocky canyons in the region, with surveys of similar habitats elsewhere in Australia often finding a high percentage of un-described species (Poore et al. 2008). For example, recent surveys in 2009 are the first to collect samples of invertebrate megafauna from deeper than 1800 m around Australia (Williams et al. 2009a)). Some work has been conducted off southeast Australia. Surveys were recently undertaken in deep-water marine reserves within the “Southeast Commonwealth Marine Reserve Network” (Department of the Environment 2008) that were gazetted in 2007 as part of the “National Representative System of Marine Protected Areas” (Williams et al. 2009b). Off East Gippsland, areas of rocky reef are exposed from muddy sediments on the steep slopes of “Big Horseshoe Canyon”, part of the Bass Canyon system. The area is the largest canyon that has been sampled in the south-east for benthic biodiversity (Williams et al. 2009b). This canyon system, at a depth of ~1500 m, has a total area of ~319 km2 (Williams et al. 2009b), and the majority of the diverse, abundant, sessile megafauna in the canyon is associated with these exposed hard surfaces (Kloser et al. 2001). Percentage cover of “rich fauna” as determined from underwater video peaked at 200-300 m (Williams et al. 2009b). Organisms include diverse, abundant, filter-feeding species, such as Temperate reefs literature review 17 dense beds of large sponges and the stalked crinoid Metacrinus cyaneus, and numerous species of octocoral (especially gold corals). This site is the type locality for M. cyaneus and it is the only known location of the species off southeastern Australia (Kloser et al. 2001; Williams et al. 2009b). Above 600 m fisheries are important in this area (Harris 2007). In contrast, the Zeehan canyon complex to the south-west of Victoria consists of mostly soft sediment substrates, with few exposed rock surfaces (Williams et al. 2007). Canyons in this area exist every ~7 km and include fisheries for several species including blue grenadier, crab, ling and rock lobster (Harris 2007). According to Harris (2007) “conservation values include links between canyons, ocean currents, upwelling processes and flows through Bass Strait”. Temperate reefs literature review 18 Several canyons in south-east Australia are the locations of the largest known aggregations of feeding and spawning fishes in the South-East Fishery region (Williams and Kloser 2004). For example, the Zeehan Canyon complex provides “areas for high-order predator foraging and blue grenadier spawning” (Harris 2007). Elsewhere, plankton and nekton have been found to be elevated in and around canyons, in addition to high densities of megafauna (Beaman et al. 2005). The use of abiotic classification of habitats to infer levels of biodiversity in these deep systems is a contentious issue (Harris et al. 2009; Williams et al. 2009a; Williams et al. 2009b), which indicates how little is currently understood about assemblages in these systems. Ecological and physical environmental drivers Major environmental factors influence reef communities in Victoria. Womersley and King (1990) split these factors into four key groups: roughness and complexity, and shading (Womersley and King 1990; Underwood and Chapman 1995; Little et al. 2009). Dynamic factors: Three features of the tides affect intertidal reef communities: the rise and fall of the tide, variation between neap and spring tides and daily progression of the tides (Underwood and Chapman 1995; Little et al. 2009). Tide - period and amplitude, cycles Water movement – currents, upwelling, surf and wave action, storms Wind – effect on tide, water movement, and humidity Physical factors Light – quality, quantity, periodicity Sea temperature stratification Air temperature (in conjunction emersion)– variation, range Relative humidity Rainfall Substratum – variation, range, with Chemical factors Salinity Nutrients Availability of gases (O2 and CO2) pH Pollution Biotic factors Competition for space, light, nutrients etc Grazing/predation Epiphytism Symbiotic relationships Intertidal reefs Tides are, perhaps, the most important environmental factor causing gradients across intertidal reefs. The direct effects will be modified by factors such as wave action, air temperature, humidity, freshwater run-off, desiccation stress, rock type, aspect, surface Waves cause major variation in assemblage structure of intertidal reef communities from exposed to sheltered areas (Underwood and Chapman 1995). The importance of the magnitude of wave exposure in shaping community structure has long been recognised (Lewis 1964; Stephenson and Stephenson 1972), with comparisons and contrasts made between exposed and sheltered intertidal reefs (e.g. Seapy and Litter 1978). A diverse range of other factors and processes influence assemblage structure and a variety of work has been carried out worldwide that quantifies how these interact with each other. For example, while molluscan grazers may impact algal cover and shape sessile assemblage structure at local scales, the availability and supply of algal propagules is also important and may in turn be partially driven by processes at broader spatial scales such as benthic-pelagic coupling and oceanic currents (Schiel 2004; Underwood and Chapman 2007; Little et al. 2009). At local scales, gradients can be seen in the relative importance of physical and biological factors on intertidal reefs. Physical factors, such as desiccation stress, generally increase in importance up the shore, while biological factors such as competition and predation increase in importance down the shore. Towards the top of the shore where physical stress is high, algal and sessile invertebrate cover is low, while towards the bottom of the shore where physical stress is low, percentage cover of algae and sessile invertebrates is high. In the mid shore, where physical and biological stress levels may be of equal importance, a bare zone is often found. In this zone, there is typically a low cover of algae due to a mixture of desiccation stress and Temperate reefs literature review 19 grazing; competition for free space is therefore usually low (Connell and Gillanders 2007; Little et al. 2009). There is a large body of research that focuses on the importance of disturbance in the structuring of intertidal reef assemblages. Disturbance is important, as free space is a limiting factor at some tidal heights on intertidal reefs and the creation of free space can lead to changes in the structure of assemblages (Little et al. 2009). Initially this research concentrated on predation as a key driver (Paine 1966) but this was followed by a general recognition that any disturbance that created space was fulfilling the same function (Connell and Gillanders 2007; Little et al. 2009). The magnitude and timing of disturbance events such as storms, ice scour and boulders overturning can create patches of space that allow new individuals to recruit and may lead to a complex mosaic of patches at various states of succession (Connell and Gillanders 2007; Little et al. 2009). On some shores, the impact of disturbance events may be relatively predictable, for example low on shores that have high algal cover (Connell and Gillanders 2007). Another key factor that affects the ecology of intertidal reefs is the arrival of propagules or “supply side ecology” (Underwood and Chapman 2007). Planktonic dispersal and survival of propagules is highly variable, and consequently, spatial and temporal variability in the number of propagules arriving is also highly variable. The interactions between these new recruits and the existing assemblage will vary greatly depending on this “patchiness” in larval supply (Underwood and Chapman 2007). Apart from supply of propagules, other bottom up processes relate to the interaction of coastal oceanography with intertidal communities, for example the effect of coastal upwelling on the supply of phytoplankton, particulate matter and nutrients to intertidal assemblages (Menge 2000). Top down and bottom up processes can interact to influence the dynamics of communities on intertidal reefs (Menge 2000). The following sections examine research that has been conducted locally. Top-down / disturbance processes In Victoria, several studies have been conducted that examine natural disturbance on intertidal reef communities. For example, Bennett (1990) examined disturbance within beds of the commonly occurring alga Hormosira banksii, and Temperate reefs literature review 20 several other authors describe the effects of very hot days combined with afternoon low tides on this species (Keough and Quinn 2000; Bellgrove et al. 2004) and mobile gastropods (Parry 1982). Parry (1982) also described predation on intertidal limpets by oystercatchers and wrasse. The majority of disturbance studies in Victoria have, however, been focused on anthropogenic disturbance events, such as the recreational use of reefs and the effects of trampling and the harvesting of invertebrates for food or bait (Addison et al. 2008) or the effects of pollution (Bellgrove et al. 1997; Hindell and Quinn 2000). In a study that has collected data from 65 intertidal reefs across Victoria, a number of bioassessment methods have been tested to determine their ability to detect impacts on reef assemblages (O'Hara et al. in press). Human impacts on rocky shore communities have been chronicled in Victoria, and appear to take two main forms: the collection of seafood from the intertidal zone; and trampling of organisms by visitors walking on the shore (Povey and Keough 1991; King 1992; Keough et al. 1993; Keough and Quinn 1998; Keough and Quinn 2000; Addison et al. 2008). Such impacts are well known and have been shown elsewhere in Australia and internationally (Underwood 1993; Fletcher and Frid 1996). Human predation (collecting) Local studies within Port Phillip Bay, focusing on the collection of gastropods from the intertidal zone, have demonstrated that the mean size and abundance (one species only) of target species were reduced at an impacted site compared to protected sites nearby (Keough et al. 1993; Keough and Quinn 2000). The indirect effects of this type of human predation have been found to be subtle and related mainly to the abundance of microalgae. For example, frequent removal of cellanid limpets can result in an increase in microalgal densities while removal of nerites results in a decrease in microalgae (Sharpe and Keough 1998). Such removals may have more complex effects than currently appreciated, and a more in depth examination is required. For example, it has been suggested that removing the largest individuals from a population of the limpet Cellana tramoserica may eliminate the bulk of the reproductive effort of this species (Parry 1982), which may in turn impact community structure on the targeted shore as well as nearby areas. There is almost no ecological information available about another main target species in Victoria, Turbo undulatus, and so it is impossible to speculate what the effect of its removal might be (Keough and Quinn 2000). Other studies undertaken on the Victorian coast at shores visited less frequently have found little evidence to suggest there is an impact of harvesting (Keough and King 1991; King 1992), although Addison et al. (2008) suggest that shellfish collections at Sorrento may have a broader impact on intertidal assemblages. There have been no studies on the harvesting of other taxa for bait in Victoria, although in New South Wales there is some evidence that the removal of other taxa such as crabs and the tunicate Pyura stolonifera is unsustainable (Fairweather 1991; Underwood 1993). This may also hold true in Victoria, where fertilisation success in isolated spawning individuals (>2 m apart) of P. stolonifera at Barwon Heads has been shown to be limited by a scarcity of sperm (Marshall 2002). Removal of individuals may therefore directly reduce reproductive capacity within local populations. Fisheries Victoria is currently liaising with local communities to determine which species they preferentially target on intertidal reefs; this information could be used to direct future research examining this type of disturbance. Trampling The other main disturbance caused by the recreational use of reefs is trampling. This has been recognised as an issue of concern in Victoria (Carey et al. 2007). There is evidence that trampling can impact intertidal reef assemblages both in Victoria (Povey and Keough 1991; Keough and Quinn 1998) and in other parts of the world (Schiel and Taylor 1999; Benedetti-Cecchi et al. 2001). Studies undertaken within Port Phillip Heads Marine National Park have found that trampling produced marked effects in two major habitats. In beds of coralline algae, the effects were short lived, but in beds of Hormosira, algal cover was greatly reduced and the number of grazing molluscs was increased in trampled areas and recovery was still not complete >400 days later (Povey and Keough 1991). This pattern was also evident in a separate, longer-term study, where two of three sites were able to recover between trampling events whilst a third site did not recover (Keough and Quinn 1998). Keough and Quinn (1998) propose a conceptual model where Hormosira can be likened to a keystone species (Paine 1995) whereby they provide damp, shady refuges for some species but also reduce the cover of microalgae - the main food source for a number of gastropod grazers. The mobile herbivores emigrate in response to increasing cover of Hormosira and when Hormosira cover is reduced, microalgal abundances increase resulting in increased densities of grazers. The authors of this study were unable to determine why one site showed no recovery over the sixyear study but they discuss the potential ramifications of sites having different levels of resilience and the management options associated with this. A management program within the Marine National Park system will be targeting trampling as a hazard and this may be able to provide data on which shores are more, or less, resilient to trampling (Carey et al. 2007). Pollution The release of pollutants is another major type of disturbance event that impacts assemblages on intertidal reefs in Victoria. Reefs within Port Phillip Bay are more likely to be impacted by a range of toxicants due to their proximity to urban and industrial areas, harbours, ports and shipping channels. There have been numerous studies documenting the superimposition of male sexual characteristics on female gastropods following exposure to TBT (tributyltin) -based antifouling paint since this was first demonstrated (Bryan et al. 1987; Gibbs et al. 1987). Foale (1993) described the incidence of imposex in the whelk Thais orbita at sites around Port Phillip Bay and found that the incidence of imposex corresponded strongly to the proximity to harbours or marinas although mean body burdens of TBT were not particularly high. The reduction in reproductive potential of intertidal predators such as whelks has the potential to impact the structure of intertidal reef assemblages (Spence et al. 1990). Studies of intertidal reef assemblages affected by TBT have not been undertaken in Port Phillip Bay, although Parry (1982) considered predation by whelks to be a minor cause of mortality in the four species of limpet he studied at a San Remo shore. A recent study (Rees et al. 2001) found that the severity and extent of imposex has been reduced except at locations that were adjacent to major ports or certain harbours. It is possible that in some areas sediments are providing a source of TBT although no data exists on this in Port Phillip Bay. Other toxicants, such as copper, have also been shown to cause imposex in certain species (Nias et al. 1993). No specific research on the effects of other toxicants on intertidal reef assemblages Temperate reefs literature review 21 within Port Phillip Bay or coastal Victoria have been conducted to our knowledge. Bottom-up / supply side processes Pollution A form of pollution that primarily has a bottomup effect through the elevation of nutrients is the discharge of sewage effluent. The majority of studies considering the effects of sewage effluent on intertidal reef assemblages in Victoria have been undertaken around Boags Rock. However the Western Treatment Plant also discharges secondarily treated sewage effluent from Melbourne into Port Phillip Bay. Most of the studies considering the impacts of this discharge have been focused on the predominantly soft sediment environment in the vicinity of these discharges. Axelrad et al. (1981) sampled the macrophyte assemblages at three basalt reefs within the Bay and found that the intertidal zone at Werribee was characterized by Centroceras clavulatum throughout the year and Ulva lactuca and Enteromorpha compressa in summer / autumn. Blooms of the blue green alga Cladophora sp. were also observed. We are not aware of more recent studies specifically aimed at the impacts of sewage effluents within Port Phillip Bay. Long-term monitoring of the macroalgal assemblage around the Boags Rock discharge found that fewer species were recorded at sites closer to the outfall, as well as a loss or reduction of Hormosira coupled with an increase in algal turfs and the polychaete Boccardia proboscidea adjacent to the discharge (Brown et al. 1990). These patterns are considered typical of those that have occurred around sewage discharges throughout the world (Littler and Murray 1975; Fairweather 1990). There is no clear consensus to explain the loss of H. Banksii (or other dominant brown algae around the world) following exposure to sewage effluent (Bellgrove et al. 1997). At Boags Rock there was no evidence that the sewage effluent detrimentally affected the availability of propagules or macroalgal recruitment (Bellgrove et al. 1997). There were, however, very high densities of propagules of opportunistic taxa such as Ulva and Enteromorpha and high recruitment of these taxa at polluted sites. Other studies have found that high concentrations of treated sewage effluent inhibit zygote germination and embryo growth of Hormosira (Doblin and Clayton 1995) and so propagule supply and recruitment may still be important in the loss of Hormosira in sewage affected areas. Temperate reefs literature review 22 It is likely that the small dispersal shadow of Hormosira, combined with potential impacts on establishing zygotes, will limit the reestablishment of this species in areas adjacent to sewage discharges (Bellgrove et al. 1997) Another study undertaken at Boags Rock has found that the recruitment of the intertidal mussel Brachiodontes rostratus was facilitated by the secondarily-treated effluent while shell growth was reduced and mortality of larger individuals was increased (Hindell and Quinn 2000). This study also discussed the implications for intertidal fauna that use the habitat created by mussel clumps. Larval supply / recruitment It is well recognised that a vital consideration in rocky shore ecology is variability in recruitment in both space and time. Recruitment depends on larval supply, local hydrodynamics, larval behaviour, predation / herbivory and the availability of adequate resources to allow settlement and survival and so recruitment to a population. Quinn (1988) undertook an experimental study to investigate the importance of conspecific adults, macroalgae and height on the shore to recruitment of the intertidal limpet, Siphonaria diemensis. In this study there was no effect of the density of adults on the density of recruits or height on the shore but there was an effect of macroalgal cover; with significantly greater recruitment to areas with encrusting macroalgae. Studies of intertidal macroalgal species in Victoria have concluded that while recruitment cannot be predicted directly from the supply of propagules, the two processes are linked (Bellgrove et al. 2004). Results indicate that while pre- and post-settlement processes are likely to influence macroalgal distribution and abundance, the temporal and spatial variability in the supply and recruitment of propagules can explain much of the patchiness in macroalgal assemblages. A study on the effects of the grazing gastropod Bembicium nanum on the recolonization of algae at Aireys Inlet found that B. nanum significantly reduced recolonization of the ephemeral brown alga Scytisiphon lomentaria but had no effect on recolonization of Hormosira or the green alga Enteromorpha intestinalis (Braley et al. 1991). A separate study has found that micrograzing copepods have the potential to greatly influence the density of various macroalgal taxa, while a littorinid mesograzer may reduce the both the density and number of macroalgal taxa (Bellgrove 1998). This study also found that two limpets studied (Patelloida latistrigata and Cellana tramoserica) did not have any significant effects on the densities of macroalgal taxa recruiting (Bellgrove 1998). This contrasts with studies at other locations that have shown rapid colonisation of foliose algae following the exclusion of patellid limpets (Hawkins 1981; Underwood and Jernakoff 1981). There have also been a number of studies specifically focused on Hormosira. While this species has a broad distribution throughout south-eastern Australia, dispersal of propagules is thought to be limited (Bellgrove et al. 1997; 2004). However, investigations into the occurrence of ‘outbreeding depression’ at a regional scale led researchers to speculate that Hormosira may be capable of longer distance dispersal than previously thought (McKenzie and Bellgrove 2006). Detached fronds of Hormosira have been shown to be capable of releasing gametes for up to 8 weeks following detachment and floating fertile fronds may be an important mechanism for facilitating longdistance dispersal in this species (McKenzie and Bellgrove 2008). Further studies investigating genetic diversity over the broad-scale distribution of Hormosira using genetic markers may give valuable insight into gene flow and long distance dispersal and are already underway. In a study that quantified species richness of invertebrates on 11 rocky intertidal shores separated by a biogeographical barrier (Ninety Mile Beach), it was revealed that species richness and species composition did differ on each side of the barrier and that the distribution of species was not related to their potential for dispersal (Hidas et al. 2007). The three species studied with direct development were found on both sides of the barrier, while seven of the eight species restricted to one or other side of the barrier had planktonic larvae. All species present on Red Bluff, an isolated rock platform, did, however, have planktonic larval stages. In contrast, a study looking at the mitochondrial DNA of the highly-dispersive intertidal gastropod Nerita atramensota found a biogeographical disjunction that was unrelated to either the Coorong or Ninety Mile Beach; instead, Wilsons Promontory was the point of disjunction (Waters 2005). The authors concluded that this sharp biogeographical disjunction was in marked contrast to the species’ high dispersal abilities and supports other studies that have noted that Wilsons Promontory may have considerable biogeographic significance for Australia’s southern temperate region (O'Hara and Poore 2000). An ongoing study looking into the connectivity of intertidal gastropod molluscs with varying dispersal potentials within marine national parks is currently comparing recruitment patterns of gastropods in Marine Protected Areas (MPA) and non MPA sites in Port Phillip Bay and the central coast of Victoria (Bathgate pers. comm.) Nutrients The supply of nutrients is considered another important bottom-up process and to date there has been very little attention in Victoria to the impacts of nutrients on rocky shore ecology. There have been studies that consider the impacts of sewage discharges on intertidal reef assemblages (see discussion above) but it is not always easy to separate the effects of increased nutrients from other aspects of the discharge (freshwater, suspended solids, toxicants etc.). A manipulative study has been undertaken at Point Nepean and found no effect of adding nutrients or removing grazers (separately or together) on the algal assemblage monitored (Baker 2006). This finding is in stark contrast to work in other parts of the world (Thompson et al. 2000; Bokn et al. 2003) and other parts of Australia (Underwood 1984; Gorgula 2004) and may relate to the time of year the experiments were undertaken (summer) when propagule supply was likely to be low (Clayton 1990; Bellgrove et al. 2004) and physical stress (heat) was high. A manipulative study undertaken in the shallow subtidal in south Australia found that increasing nutrients had no effect on algal assemblages in the presence of canopies and, in the absence of canopies, only had an effect on algal assemblages in the presence of grazers (Russell and Connell 2005). They attributed these complex interactions to the fact that the area of coast studied had few grazers (weak topdown control by grazers) and was relatively oligotrophic. There is some evidence that the coastal regions of Victoria are also under weak consumer control compared to eastern Australia (Beovich and Quinn 1992; Bellgrove 1998; Sharpe and Keough 1998; Burton 1999) and this may affect the response of assemblages to elevated nutrients. Ambient nutrient levels measured at Cheviot Beach (Parry and Restall 2007) were very similar to those cited in the South Australian study and considerably less than nutrient concentrations in New South Wales (Russell and Connell 2005). Further Temperate reefs literature review 23 studies are required to investigate how nutrient supply might affect recruitment of Hormosira and other algal species, grazer control, competitive interactions and responses to anthropogenic disturbances such as trampling. Competition Studies of competition on intertidal reefs in Victoria have primarily focused on exploitative intra and interspecific competition of herbivorous gastropods. Studies undertaken at San Remo have found that densities of Austrocochlea constricta and Bembicium nanum were not restricted by interspecific competition for food, although intraspecific effects did occur in summer and autumn due to a reduction in food supply (Quinn and Ryan 1989). Quinn and Ryan (1989) also found that growth was not a fixed characteristic in Siphonaria diemensis and can change rapidly in response to variations in food supply; growth decreased with increased densities of conspecifics, and shell growth and tissue weight were reduced in an experimental treatment with reduced food compared to a normal food treatment. Beovich and Quinn (1992) found no evidence that Siphonaria lomentaria were food limited by Cellana tramoserica in winter and spring at this site. Studies on basalt rock in Port Phillip Bay have found that Cellana tramoserica individuals are strong exploitative competitors for food resources and when exposed to high densities of conspecifics experience reduced growth and increased mortality (Wright 1989; Marshall and Keough 1994; Merory 1997). These results are consistent with studies from other parts of Victoria (Parry 1982; Burton 1999) and New South Wales (Creese and Underwood 1982). Marshall and Keough (1994) also found an asymmetry in the competitive ability of cellanid limpets, with smaller individuals able to out compete larger individuals. They speculated that smaller limpets were able to access a food supply within the rough rock surface that larger limpets with their larger radulae were not able to access. This hypothesis was later tested by repeating the experiment on basalt rock at Williamstown and limestone rock on the exposed Point Nepean shore (Keough et al. 1997). This study confirmed predictions that asymmetry in competitive abilities of limpets would not be apparent on a smoother substratum and was further supported from results from another study undertaken at Point Nepean (Burton 1999). Cellanid limpets were also shown to be competitively superior to a Temperate reefs literature review 24 pulmonate, spihonariad limpet at the exposed rocky shore of Point Nepean (Burton 1999), in contrast to a previous study undertaken at San Remo (Beovich and Quinn 1992). In the Burton (1999) study and that of Keough et al. (1997) it was shown that while patterns of cellanid growth varied in response to competitive pressures, microalgal abundance did not. This is in contrast to findings from other parts of Australia (Underwood 1984) and other parts of the world (Little et al. 2009). Further investigation concluded that feeding behaviour was not important to limpet growth and mortality, and that the reasons for the observed effect of increased densities on growth and mortality at Cheviot Beach (Point Nepean) remain unresolved (Burton 1999). There is evidence that the outcomes of exploitative competition in Victoria can depend on rock type and seasonal effects of food supply, and appear to differ in some respects from similar studies undertaken in New South Wales. While there is evidence of competition for food in Victorian populations, it does not appear to be driving distributions as in New South Wales. While in some instances removal or reduction in grazer densities on Victorian shores did cause an increase in algal growth, the magnitude of effects was much smaller than observed in New South Wales and other parts of the world. Subtidal reef A wide range of factors and processes are responsible for the distribution and structure of assemblages on subtidal reefs. These have recently been reviewed in some depth (Connell 2007; Connell and Irving 2009; Wahl 2009), and the descriptions that follow below cover work that has been conducted in Victoria, with relevant examples from elsewhere also included to provide context. Physical drivers Biogeographical provinces or regions have been demonstrated to influence the distribution and composition of subtidal reef communities in Victoria. Waters et al. (in press) recently provided an a priori test for the existence of Bennett and Pope’s (1952) Maugean (southeastern), Flindersian (western) and Peronian (eastern) biogeographical provinces across southern Australia. The study quantitatively analysed distributional data from 1,500 algal species and identified the three distinct biogeographical assemblages, consistent with traditional qualitative provinces. Connell and Irving (2008) quantified the frequency and size of patches of major benthic habitat on across the southern coast of Australia and went on to show that biogeography had a fundamental influence on the patterns of abundance and composition of subtidal assemblages across regional scales. The most fundamental patterns related to (1) the proportion of rock covered by kelp forests, as related to particular functional groups of herbivores, and (2) the small-scale heterogeneity that characterises these forests. Prior to this, O'Hara (2001) tested the assumption that habitat defined by various biological and environmental variables are surrogates for biodiversity. The study collected and analysed benthic floral and faunal samples from subtidal reefs at 23 sites along the coast of Victoria, and determined that habitats defined by dominant vegetation, and to a lesser extent region, supported consistent floral and faunal assemblages. For each sample, habitat parameters were recorded including dominant vegetation, geographic region, depth, rock type and exposure. Although physical variables were determined to have little emphasis as drivers, the author acknowledges that the experimental design was probably not an appropriate test for the effect of depth range, rock type and exposure on biodiversity. In contrast, Edmunds et al. (2000) specifically set out to establish which physical variables influence the distribution and composition of reef communities. The study involved surveying seven subtidal (8 – 18 m) sites between Cape Schank and Wilsons Promontory to provide a variety of reef substratum types, recording biological (density of macroinvertebrates, canopy forming plants, cover and biomass of understorey macroalgae) and physical (substratum lithology, relief, interstitial space, and complexity, depth, exposure, aspect, distance from shore, slope, longitude and sediment cover) variables. Results of the study found that many reef inhabiting biota were closely associated with particular physical variables. Invertebrates were closely associated with combinations of substratum structure (i.e. interstitial space, complexity, slope and relief), depth and longitude whilst aspect and exposure were also of some importance. The slope of rock has been described as the most striking feature influencing the composition of assemblages of rocky subtidal systems by Connell (2007). The position of rock affects key physical processes such as light, flow and sedimentation, as well as biological processes including consumer foraging, competition, facilitation and recruitment. Edmunds et al. (2000) found algae had less correspondence with physical data, but exposure, depth and reef slope were reasonably good explanatory variables. Substratum type lithology as a single variable was not found to be a major determinant, although some species occurred predominantly on one substratum type. The authors go on to state that the relationships between variables measured do not necessarily imply causality, but the physical-biological relationships described are similar to those found in studies of temperate reef assemblages in New Zealand (Choat and Schiel 1982; Choat and Ayling 1987). In New Zealand, depth and exposure have been shown to play an important role in algal and echinoid distribution (Choat and Schiel 1982), and echinoid and algal habitat have been shown to play an important role in determining associated fish fauna (Choat and Ayling 1987). These findings have been reiterated in Australian studies with depth and wave exposure shown to influence the distribution and composition of subtidal temperate reef communities in southern Australia. Edgar (1984) Temperate reefs literature review 25 described benthic floral and faunal assemblages along the eastern, southern and western Tasmanian coasts and related them to a subtidal zonation pattern of wave exposure and depth. Results of the study found that major assemblages of benthic organisms within the same cool-temperate biogeographical provinces (Maugean and Flindersian), can be predicted reasonably accurately by reference to wave exposure and depth. Coleman et al. (2007) found depth to be a strong driver of patterns of mobile invertebrates in kelp forests on the temperate coast of Western Australia. Their study found a greater abundance and richness of common taxa in holdfasts from shallow relative to deep waters. Such differences in the distribution of species with depth can also be seen for a variety of reef fish. For example, the reef fish Achoerodus viridis has been shown to decrease from shallow to deep areas of reef and from inner to outer areas of estuaries, although large individuals increased in numbers on more exposed coastal reefs (Gillanders 1997a; Gillanders 1997b). Fish found on rocky reefs in New South Wales, in particular labrids, have been found to show distinct patterns in diversity and abundance in relation to wave exposure and depth (Fulton and Bellwood 2004). Few species were found to be abundant in exposed shallow habitats, while several species were restricted to sheltered habitats (Fulton and Bellwood 2004). Seasonal fluctuations in water temperature may also result in changes in the abundance of fishes on temperate reefs (Parker Jr 1990). Studies describing marine biodiversity in Victoria have found depth and geography to have the strongest influence on the composition of communities. Ferns et al. (2000) described temperate reef biodiversity through mapping and analysis of biological data and concluded that depth is an important factor in the distribution of dominant biota because of localized conditions such as substratum topography/complexity and wave exposure. Victorian temperate reef community Marine Habiat Class (MHC) attributes generally differ between shallow (0 - 2.5 m), moderate (2.5 – 20 m) and deeper (>20 m) subtidal depths. Broad patterns of algal species zonation along the depth gradient have also been shown in Victoria (Sanderson 1997): Hormosira banksii is the dominant species observed on intertidal rock platforms; Durvillaea potatorum with mixed brown and green algae inhabits the seaward edge of intertidal rock platforms; Phyllospora Temperate reefs literature review 26 comosa occurs in large beds at depths of ~3–5 m; at exposed sites, Ecklonia radiata is typically observed at depths >7 m and often growing with P. comosa; and, giant kelp Macrocystis angustifolia (an indicator species for the Maugean biogeographical province) occurs at Port Phillip Heads MNP - Point Lonsdale and at Point Nepean. In South Australia, Connell (2003a; b; 2005) has investigated the importance of physical processes such as light penetration and sedimentation in structuring benthic communities, and a number of studies elsewhere have also shown that subtidal assemblage distributions vary due to a variety of physical factors including light availability, wave action and storm disturbance (Dayton et al. 1984; Schiel and Foster 1986). Connell (2003a) tested the hypothesis that different temperate reef-algal habitat types will converge to become like those under Ecklonia radiata if subjected to the same low light and accumulation of sediment without the presence of E. radiata. The study found habitats did not converge under treatments. The author concludes that canopies place strong constraints on the presence and abundance of many taxa, but not encrustingalgal habitats which beneficially coexist as understorey. Connell (2003b) then went on to investigate why many sessile invertebrates are anomalously absent from understorey communities. A series of experiments were applied to partition both positive and negative habitat modifying effects by kelp for sessile invertebrate recruitment. The key finding of the study was that a reduction of light intensity and removal of sediment by canopies acted to facilitate recruitment, but physical abrasion by the canopy acted as a negative force to overpower these positive effects for recruitment of sessile invertebrates. Connell (2005) experimentally separated the positive and negative effect of light penetration and sedimentation on the assembly and maintenance of three subtidal habitats that characterise much of the world’s temperate coastline: encrusting coralline algae, articulate coralline algae and filamentous turf-forming algae. The study found shade to cause a positive effect for encrusting coralline algae by facilitating the retention of space without overgrowth; however, it caused a negative effect for articulate coralline and turfforming algae by restricting growth and space persistence. Sediment deposition caused a negative effect on encrusting coralline algae, a positive effect on articulated coralline algae and a neutral effect on turf-forming algae. This finding explains the presence of articulated coralline and turf-forming algae on humandominated coasts following the loss of canopy forming algae on reefs with heavy sedimentation. The studies demonstrate the key role of physical factors associated with habitatforming canopy algae, particularly in relation to human-dominated coasts with heavy sedimentation. Another physical driver of subtidal reef communities is the presence of oceanographic features such as upwelling that can alter regimes of variables such as nutrients and temperature. For example, Butler et al. (2002b) states that the Bonney upwelling area contains “distinct colder water flora; rich assemblages of sessile filter feeders such as sponges, bryozoans and corals; feeding grounds for seabirds, fishes, whales and other higher order predators such as fur seals and penguins; and a productive fishing ground for rock lobster, sustaining a relatively large fishing industry”. Butler et al. (2002b) described how limestone reefs in the area are often covered by dense assemblages of molluscs, sponges, bryozoans and red algae, with upwelling related assemblages of bryozoans, sponges and axoozanthellate coral on the shelf edge and upper slope, and off Portland, diverse cyclostome bryozoans are found on the deep shelf. Ecological drivers Habitat structure Ecological components of temperate reefs have been shown to influence the distribution and composition of subtidal reef communities in Victoria. Algae, in particular canopy forming species such as kelps, act as foundations and strong ecological drivers to entire subtidal systems because they provide important food and habitat structure for other organisms on the reef (Duggins 1989; Keough and Butler 1996). Variation in the configuration of subtidal algae in southern Australia and northern New Zealand (e.g. monotypic or mixed stands of algae) may influence the composition and abundance of associated organisms (Goodsell et al. 2004) such as understorey algae and sessile invertebrates (Irving et al. 2004). O'Hara (2000b; 2001) used multivariate analysis and determined that dominant vegetation is a primary ecological driver of subtidal reef habitats. The study concluded that larger kelps (e.g. Phyllospora, Ecklonia, and Macrocystis) supported a relatively species-poor epiphytic community compared with Cystophora and Sargassum species. However, Ecklonia and Macrocystis had large branching holdfasts that provided shelter to many larger cryptic animals, and provided stable substratum for many sessile invertebrates and smaller algae. Algae can influence the presence of other marine biota such as fish and invertebrates (Andrew 1993; Edmunds et al. 2006a). Variation in algal cover and other biotic factors can show considerable variation spatially and temporally (Dayton et al. 1984; Schiel and Foster 1986), and this can subsequently be seen in the distribution and abundance of associated invertebrate and fish species (Russell 1977; Bodkin 1988; Holbrook et al. 1990a, b; Schmitt and Holbrook 1990a; Anderson 1994). A large body of work has been conducted in California, USA on temperate reef systems. Canopy-forming kelps such as Macrocystis pyrifera can directly affect the densities of fish species that use the kelp as a nursery area and/or adult habitat (Ebeling and Laur 1985; Bodkin 1988; Holbrook et al. 1990a; Carr 1994).The effects of increases or decreases in different types of algal community have been found to be strongly related to the resources required by different life history stages of fishes (Holbrook et al. 1990a), as well as to the differing needs of individual species of fish (Holbrook et al. 1990b). For example, positive effects have been chronicled for species that use kelp as a nursery ground or adult habitat; whereas negative effects can be seen for fish that rely on understorey species that are impacted by shading effects and which may serve as important sources of food (Holbrook et al. 1990a; Schmitt and Holbrook 1990a; b). While work on temperate reefs in California, suggest that the distribution and abundance of canopy and understorey algae may partially explain spatial variation in the species composition of fish recruitment among temperate rocky reefs at the local level (Bodkin 1988; Carr 1989), heterogeneity of other habitat features, e.g. the presence of sand patches, is also likely to drive spatial variability in the distribution of reef fishes, particularly at small spatial scales (Garcı´a-Charton and Pe´rezRuzafa 2001). Macroalgal structure cannot, therefore, necessarily be used as an indicator of recruitment strength for algae associated fish on reefs (Anderson 1994). Effects of habitat forming biota are not just limited to plants, e.g. sponge assemblages, have Temperate reefs literature review 27 also been shown to directly influence the distribution of reef fish in New South Wales (Curley et al. 2002) as well as other areas, such as the Mediterranean (Sanchez-Jerez et al. 2002). Herbivory and Grazing Herbivorous fish can make up to 47% of the fish species present on some Victorian subtidal reefs (Jones and Andrew 1990), and these species are often associated with their preferred algal food (Port of Melbourne Corporation 2007c). For example, fish species such as herring cale Odax cyanomelas feed almost exclusively on patches of Ecklonia radiata and are more abundant on the southern shallow reefs where these species are more dominant (Jones and Andrew 1990). The Victorian scalyfin Parma victoriae feeds in areas where kelp is absent and red algal turfs are present (Jones and Andrew 1990). Territorial herbivorous fish such as P. victoriae and O. cyanomelas may affect algal distribution and abundance by (1) direct feeding effect due to consumption of algae, (2) weeding out of algae to promote growth of preferred algae and (3) effects due to exclusion of other herbivorous fish. Herbivorous fish are generally found to have a localised impact on algae where as grazing urchins like Centrostephanus rodgersii appear to have a major effect on spatial patterns in the distribution of macroalgae (Jones and Andrew 1990). Sea urchins are described throughout the literature as key components of temperate reef ecosystems as they graze on algae and modify habitat, influencing the distribution and composition of subtidal reef communities (Australian Government 2005). Several species of urchins exist in Victoria but only the black urchin Centrostephanus rogersii causes ‘urchin barren’ or ‘white rock’ habitat which is devoid of macroalgae (Australian Government 2005). Urchin barrens are restricted to the far-east of Victoria where C. rogersii is present in high abundance (Keough and Butler 1996; O'Hara 2000b; Ferns 2003; Department of the Environment and Heritage 2005). Several studies have been conducted on the creation, maintenance and effect of urchin barrens elsewhere in southern Australia and New Zealand (Andrew and MacDiarmid 1991; Andrew 1993; Andrew and O'Neill 2000). In Victoria, urchin barrens have been described at Cape Howe MNP (Ball and Blake 2007b). The presence of C. rogersii barrens represents one of the major differences between the Perionian and Temperate reefs literature review 28 Flindersarian biogeographical provinces (Connell 2007) C. rodgersii and another barren forming urchin Evenchinus chloroticus appear to have a major impact on habitats, and patterns in the abundance and foraging of other species. Herbivorous fish species such as Odax and Parma victoriae were found to be influenced by the grazing of both of these urchin species, but the authors conclude that it is unknown if this finding is the representative of all sea urchinfish species interactions (Jones and Andrew 1990). Andrew and Stocker (1986) studied the microhabitat, occupancy, dispersion and movement of E. chloroticus. The study found sea urchins were positively associated with encrusting coralline algae but movement with regards to algae needs to be further investigated. Andrew (1993) investigated the effect of availability of shelter on the foraging behaviour of C. rogersii, and on the abundance of invertebrates and algae. The study found that urchin recruitment was not restricted to high shelter areas, but barren creation was heavily reliant on shelter and urchin grazing caused reductions in the density of foliose algae and limpets. Andrew and O'Neill (2000) mapped shallow subtidal habitats in New South Wales and estimated the coverage of C. rogersii barren habitat. The study found that the extensive coverage of the barren habitat in New South Wales is likely to limit the productivity of the abalone industry and the development of a sea urchin fishery may have large impacts on habitat representation on near shore reefs. A commercial fishery is currently developing for sea urchins in Victoria with harvesting of the white sea urchin Heliocidaris and C. rogersii recording a catch valued at $191,199 in 2003/04 (Australian Government 2005). In New South Wales, Gillanders and Kingsford (1998) found small Achoerodus viridis in greater numbers in kelp beds than in adjacent urchin barrens, although the species appeared to be flexible in its use of habitats on reefs, suggesting that populations would survive readily if more urchin barrens were created in an area . In north-eastern New Zealand, some species of reef fish have shown similar patterns, while others were more closely linked to kelp or urchin barrens (Anderson and Millar 2004). Parma microlepsis in NSW have been shown to exhibit exactly the opposite pattern (Holbrook et al. 1994). Larval supply/recruitment Like intertidal habitats, larval supply, connectivity and recruitment are key processes structuring communities on sub-tidal reefs (Keough and Swearer 2007). Recruitment processes in blacklip abalone, Haliotis rubra, were related to regional hydrodynamics in eastern Victoria (McShane et al. 1988). The study suggested that dispersal of abalone larvae is primarily local. Results indicated that local reef topography could sufficiently attenuate currents for larvae to stay on the parent reef for the full 3 -7 day pelagic period. Hunt (2007) investigated the ecological determinants for recruitment in a reef-associated planktivorous fish in Port Phillip Bay, the southern hulafish Trachinops caudimaculatus. The study surveyed the density of fish populations and various microhabitat characteristics and analysed them together to detect relationships. Microhabitat characteristics explained 85% of the spatial variation in recruit density, with food accessibility characteristics in the form of high plankton supply and low suspended sediment being the most important for recruitment. Jenkins and Wheatley (1998) studied the recruitment of fish in shallow sub-tidal habitats in Port Phillip Bay and found that while most emphasis in the past has been placed on seagrass beds as nursery areas for juvenile fish, reef-algal habitat showed similar levels of recruitment to seagrass habitats for a number of species such as King George whiting, Sillaginodes punctata. Juveniles of most species commonly thought to use seagrass as a nursery area were also found to recruit to shallow subtidal reef. Exceptions were relatively specialised groups such as pipefish. Although the number of studies to date in Victoria on recruitment to subtidal reefs is limited, the are currently a number of studies underway addressing issues of larval supply, connectivity and recruitment of fish to subtidal reefs in Port Port Phillip Bay (S.E. Swearer, P.A. Hamer, pers. comm.). Competition In contrast to intertidal reefs, little research has been conducted on competition on subtidal reefs in Victoria. Competitive relationships for food and space are likely to exist between abalone and sea urchins as both co-occur in reef ecosystems and feed on drift algae (Jenkins 2004). Urchins may be better competitors where food is limiting as they can graze directly on macroalgae and create barrens where abalone do not occur (Andrew and Underwood 1992). Jenkins (2004) states that most work would suggest abalone fluctuations may be affected by urchin abundances and the reverse is less likely. However, there are suggestions that when food is not limiting abalone are superior competitors for space, such as in crevices. At Popes Eye in Port Phillip Bay, research on Parma victoriae found that territory size was related to intraspecific interactions, with the size of territories increasing considerably when neighbours were removed. No correlation existed between territory size and fish body size or age, and no relationship was found between territory size and food abundance following experimental manipulations of algal food abundance (Norman and Jones 1984). P. victoriae with larger territories do, however, have a wider variety and abundance of food types as larger territories typically include a wider range of microhabitats (Jones and Norman 1986). Predation Predation is a top-down process that has been linked to trophic cascades in subtidal ecosystems. This occurs where removal of large predators from the reef ecosystem leads to an increase in lower trophic levels that can have profound effects on the ecosystem. An example of this occurs in New Zealand where removal of snapper (Pagrus auratus) and rock lobster (Jasus edwardsii) leads to a proliferation of urchins and subsequent barren formation (Shears and Babcock 2002). When marine protected areas were declared, there was a subsequent increase in lobsters and snapper, and a corresponding growth of kelp forests (Shears and Babcock 2002). Rock lobster are considered to be a keystone species on temperate reefs in Victoria because their feeding activities can have a significant effect on the structure of reef ecosystems (Jenkins et al. 2005b). Adult rock lobsters are carnivorous and feed mostly at night on a variety of bottom dwelling invertebrates such as molluscs, crustaceans and echinoderms (Department of Primary Industries 2009b). In Tasmania, the spread of Centrostephanus rodgersii barrens may be related both to climate change (facilitating a range extension) and heavy fishing of rock lobsters allowing urchin populations to increase and lead to barren formation (Connell 2007). Temperate reefs literature review 29 The few studies of predation on subtidal reefs in Victoria have focussed on the 11 arm sea stars Coscinasterias muricata. Day et al. (1995) found that C. muricata fed predominantly on mussels and to a lesser extent other molluscs and invertebrates. Abalone were rarely eaten except where aggregations were found eating abalone when other prey were scarce. Abalone were found to have a number of behavioural responses that increased the likelihood of escaping predation. McShane and Smith (1986) recorded very high mortality of tagged abalone as a result of predation by a dense aggregation of C. muricata on a subtidal reef in Port Phillip Bay. Major commercial fisheries Subtidal reefs support the two most valuable commercial fisheries in Victoria: abalone and rock lobster. The Victorian abalone fishery commenced in 1962 and includes blacklip, Haliotis rubra and greenlip, H. laevigata species. Abalone are the most valuable commercial fishery in Victoria, worth currently about $28.5 million (Department of Primary Industries 2009a). The rock lobster, J. edwardii fishery is the second most valuable commercial fishery in Victoria after abalone. The 2008/09 catch was valued at about $14.3 million (Department of Primary Industries 2009b). After a planktonic larval stage, both abalone and rock lobster settle to a benthic rocky reef existence on coastal reefs throughout Victoria (McShane 1999; Department of Primary Industries 2009b). Abalone are found on Victorian reefs to depths of 60 m but they are most commonly found in shallow water less than 10 m deep (Harry Gorfine pers. comm, McShane 1999). Greenlip abalone are patchily distributed west of Wilsons Promontory whilst blacklip abalone have a relatively consistent distribution,, extending throughout Victoria (Harry Gorfine Pers. comm.). Southern rock lobster are found to depths of 150 metres, with most of the commercial catch coming from inshore water less than 100 metres deep (Department of Primary Industries 2009b). The abundance of rock lobster decreases from west to east in Victoria reflecting a decreasing area of suitable rocky reef habitat (Department of Primary Industries 2009b). The ecology of abalone and rock lobster has been investigated in several studies revealing relationships with biotic and abiotic features of Victorian temperate reef habitats. Temperate reefs literature review 30 Abalone A number of studies have been conducted in Victoria on abalone movement and dispersal, particularly in terms of implications for abundance and mortality estimates for stock assessment purposes. Abalone were found to move and re-aggregate after removal by experimental fishing, potentially affecting estimates of abundance (Officer et al. 2001). Another study followed dispersal of tagged abalone from experimental plots (Dixon et al. 1998). Dispersal contributed 40-60% of tag disappearance and potentially has a significant affect on mortality estimates. The magnitude of movements varied with habitat quality and is potentially affected by fishing (Dixon et al. 1998). Jenkins (2004) summarised the ecosystem effects of abalone fishing by reviewing papers that investigated interactions between abalone and the Victorian temperate reef ecosystem including feeding, competition, commensalism, predation and parasitism. Abalone appear not to have a structurally important role in the reef ecosystem due to their passive feeding on drift macroalgae (Shepherd and Clarkson 2001). Jenkins (2004) concluded that the literature suggests a low effect of abalone fishing on the ecosystem in comparison to other fishing activities, such as trawling and dredging. However, this should not circumvent rigorous experiments into the impacts of the fishery and investigation into ecological interactions. Following this conclusion, Jenkins et al. (2005a) analysed abalone/ecosystem monitoring datasets from the Abalone Assessment Monitoring program conducted by DPI and the Subtidal Reef Monitoring Program conducted by Parks Victoria/DSE to identify ecological interactions and potential indicator species for abalone. The taxon that showed positive correlation over the broadest geographical area was encrusting algae or “pink rock”, which abalone larvae are dependent on for settlement (Daume et al. 1999). Considering abalone undergo limited dispersal (Prince 2003), Jenkins et al. (2005a) described the correlation of abalone and crustose coralline algae as a reasonable expectation. Rock lobster A recent project by Ball et al. (in prep) used underwater video techniques to (1) describe critical habitat of rock lobsters and (2) identify potential interactions between rock lobster and other species. A total of twelve fixed sites were surveyed using underwater video in the western and eastern zones of the Victorian rock lobster fishery. The video habitat data was analysed with rock lobster catch data to assess interactions between rock lobsters, habitat and other species. Results for the analyses determined that distinct habitat assemblages could be linked with depth, and a number of habitat parameters were identified as being important in determining rock lobster distributions. These variables were depth, proportion of continuous rocky reef, percentage cover of P. comosa, percentage cover of understorey red algae and percentage cover of sessile invertebrates. A positive relationship was found between rock lobster and patchy reef, which may provide rock lobster with greater foraging opportunities and protection compared with continuous reef. The study concludes by stating that it only investigated the relationship between physical and biological habitat and rock lobster catches, but oceanographic influences such as wave energy and nutrient rich upwellings may also be linked to rock lobster distribution and abundance. Temperate reefs literature review 31 Deep reefs and canyons The Port Phillip Heads region contains deep reef habitat and species that are unique within Port Phillip Bay (Edmunds et al. 2006b). These habitats and the biota resident there were surveyed prior to recent channel deepening activity, primarily using video from ROVs, as were other deep reef habitats within Port Phillip Bay which included the Rip (between Point Lonsdale and Point Nepean), Schnapper Deep, Portsea Hole, Spectacular Reef and Far Side Reef (diver video), and at Wilson’s Promontory (drop video) and Point Addis (towed video) on the open coast (Edmunds et al. 2006b). This study described a variety of substrate types in the Rip and on other deep reefs within Port Phillip Bay, with deep areas of The Rip having more complex surface complexity than shallow areas (Chidgey 2006). Deep reefs in Port Phillip Heads contain a range of assemblage types, some of which are different to all other reefs surveyed within and outside Port Phillip Bay, and are mainly dominated by encrusting sponges and hydroids (Edmunds et al. 2006b). Many of the factors and processes responsible for shaping assemblages on deep reefs are the same as those that occur on shallow subtidal and intertidal reefs (see previous discussion). For example, substrate angle can effect the distribution of sponges on deep reefs, and sponge diversity also varies with depth (Maldonado and Young 1996). Edmunds et al. (2006b) discussed the biology of organisms on deep reefs in Victoria, with the assumption that this was broadly similar to species studied on shallow reefs, and described a variety of physical and ecological drivers related to the ecology of deep reefs in Victoria, particularly those in and around Port Phillip Heads. These processes often interact with each other and can therefore exert a variable influence on species found on and around deep reefs. Very little, if any, research has been conducted in Victoria on these patterns and processes and that done elsewhere in deep water has tended to concentrate on more widely distributed soft sediments (Levin et al. 2001) This is due in part to the perceived difficulties associated with sampling and conducting manipulative experiments on deep reefs. A summary of the likely factors involved in shaping the structure of assemblages are laid out in Tables 5-7 following a recent review by Temperate reefs literature review 32 Edmunds et al. (2006b) and several of these drivers are discussed below. Physical drivers As light intensity diminishes with depth, animals replace plants as the dominant life form (Keough and Butler 1996; O'Hara et al. 1999). Algae, including encrusting red coralline algae that are often found on deep reefs, is typically replaced by attached invertebrates such as sponges, bryozoans, corals and ascidians (Edmunds et al. 2006b). Recent surveys carried out on a number of deep reefs in Victoria have described how the dominant biota shows zonation with depth (Ball et al. in prep). Phyllospora comosa (crayweed) was observed to depths of ~29 m, and typically grew with the kelp Ecklonia radiate which was the dominant species observed to depths of ~ 48 m. E. radiata was the dominant species at the majority sites, while P. comosa alone or with E. radiata was an important component of the biota at a range of sites at Portland, Warrnambool, Warrnambool West, Port Fairy West and from Ocean Grove to Torquay. At 60–80 m, sessile invertebrates began to replace these dominant large brown macroalgae. Ryan et al. (2005) described how submarine canyon and shelf topography can influence physical–biological coupling. Considerable amounts of water circulate through deep water canyons due to a range of factors including temperature gradients, eddies, oceanic currents and upwelling (Breaker and Broenkow 1994). Such movements of large bodies of water have been shown to drive the movement of relatively large quantities of sediments and sand (Hume et al. 2000), which has ramifications for organisms that live in these environments. For example, high levels of organic matter in sediments may influence species richness (Curdia et al. 2004), excess sediments may cover and destroy assemblages (Okey 2003), or nutrient transport may be affected as in Perth Canyon off Western Australia where interactions occur between the Leeuwin Current and upwelling (Hume et al. 2000; Rennie et al. 2009). Interactions between topography and currents can also influence the distribution of deep reef fishes. On deep shelf areas where cold water is uplifted from slopes, areas can exist where waters are nutrient rich and key prey are abundant (Bax and Williams 2000), for example, at the head of Horseshoe Canyon where productive fishing grounds are found (Bax and Williams 2001). Strong currents may effect certain species due to their physical structure, and at a smaller scale, cracks, crevices and holes of varying sizes can provide microhabitat and influence the distribution of species in relation to water movement (Beaman et al. 2005). For example, currents in Port Phillip Heads are likely to have a greater effect on erect sponge species than encrusting species, as has been seen elsewhere (Bell and Barnes 2000; Bell et al. 2002), and may result in certain sponge species being found in encrusting rather than erect forms (Abdo et al. 2008). (Hypoplectrodes nigroruber). Schools of barber perch (Caesioperca razor) are replaced by the related butterfly perch (Caesioperca Lepidoptera) (O'Hara et al. 1999). While fish present on shallow subtidal reefs include algavores, omnivores and carnivores, those on deep reefs are typically carnivorous as algae are typically not present at depth. Although common on rocky reefs, sponges, hydrozoans, anthozoans, bryozoans, and ascidians are thought to be largely unpalatable to reef fish (Russell 1983); it is therefore likely that fish at these depths are feeding on associated mobile invertebrate fauna. Sponge assemblages are more stable on solid reef rather than boulder substrates, but are more diverse on boulders (Carballo and Nava 2007). This may be due in part to the fact that sponges on boulders can occur on surfaces at a variety of angles which produces microhabitats with varying degrees of shading and water movement. Boulders may also be more prone to disturbance, and intermediate levels of disturbance are generally thought to increase diversity (Sousa 1979). Competition Sponges in deep water have been shown to be important competitors for space (Suchanek et al. 1983) and may live for long periods (Ayling 1981), which is likely to effect the distribution of other sessile species on deep reefs/canyons such as hydroids and bryozoans. The competitive ability of sponges is also increased by their ability to regrow swiftly after injury (Duckworth 2003), and the ability of some encrusting species to grow quickly when free space becomes available yet grow slowly when it is limited and they are undisturbed (Roberts and Davis 1996). Ecological drivers Herbivory and grazing Verges et al. (2009) found that herbivory differs with depth from shallow to deep reefs and has an important effect of the vertical distribution of algae to ~40 m. In deeper areas of reefs, sessile species such as sponges provide structure and diversity, and may provide either direct or indirect resources for fish (Tissot et al. 2006). Species richness and fish densities vary from shallow to deep reefs (Love et al. 2009), and heterogeneous habitats, substrate type and complexity have been shown to influence the distribution of fish assemblages on deep reefs (Yoklavich et al. 2000; Anderson et al. 2009). Habitat structure The distribution of fish fauna is governed by biologically formed habitat structure as well as by food, and begins to change with the loss of the kelp-associated wrasses and leatherjackets, and the appearance of fishes such as boarfishes (family Pentacerotidae), splendid perch (Callanthias australis) and banded seaperch While sessile species may compete for free space, they can also have positive interactions on each other. For example, Abdo et al. (2008) found that neighbouring sponge species protected each other from environmental impacts. Larval supply/recruitment Sponges can reproduce asexually and spread through the water column or across substrates or neighbouring individuals. They can also reproduce sexually, releasing larvae, propagules or gametes into the water column. While asexual reproduction and growth may be of primary importance to sponges (Jackson 1986), dispersal and recruitment are also important in sustaining populations and shaping assemblage structure (Keough 1988; Underwood and Keough 2001). The presence of large sessile species may effect larval deposition and food availability of adjacent species by altering fluid dynamics in the immediate area (Nowell and Jumars 1984). Temperate reefs literature review 33 Knowledge gaps Recent and current efforts to map reef biota in relation to substrate and habitat type are commendable and are providing a wealth of information on local systems. However, the majority of information on reefs in Victoria provides only a “snapshot” of assemblage structure and the distribution of species at one particular time of year or sampling site. What is lacking are examinations of temporal and spatial variation in the structure of assemblages on different reef types, and in many cases more basic information on which species occur and their basic ecology and behaviour. While general patterns that shape reef communities have been examined world wide, and to some extent locally, knowledge of processes and species interactions effecting local species are less well understood. This is problematic as this information is likely to be important in helping us to understand what is shaping many of these communities. the physical and ecological processes affecting the system. While understanding what species are in assemblages and how and why they vary is important, we also need to conduct research that examines marine ecosystems in a broader geographical context across southern Australia. Victoria’s placement at the convergence of several biogeographical provinces (i.e. Flindersian, Peronian and Maugean) make it the ideal place to conduct studies on a variety of questions at these broader scales. The amount of research and level of understanding has been greatest for intertidal reefs, is lower for subtidal reefs, and is very limited for deep and canyon reefs. This is probably a reflection of ease of accessibility and logistical constraints. Research on subtidal reefs in Port Phillip Bay has been fragmentary, and there is a poor understanding of the drivers influencing the reef communities and how these differ from the open coast. Further research on the physiological and ecological drivers affecting subtidal reefs in Port Phillip Bay is required. Of special interest are the deep reef and canyon habitats of Port Phillip Heads. This area has a unique combination of deep water, strong currents, and coastal sedimentary processes. The sessile invertebrate communities are well developed but their uniqueness is uncertain. Detailed studies of taxonomy and assemblage structure are required along with research into Temperate reefs literature review 34 In the following we outline knowledge gaps that exist for each of the reef systems that we have discussed in this review. Intertidal reefs Much of the work on reefs in Victoria that has examined patterns and processes such as competition and settlement has been conducted on intertidal reefs. There are, however, still a number of knowledge gaps that include: Basic information on the biology and ecology of a range of species Quantitative examinations of spatial and temporal variation of assemblages on many shores, both in regional Victoria and within Port Phillip Bay Potential impacts of sedimentation, pollution etc on assemblages Linkages with adjacent assemblages from different habitats - e.g. how highly mobile predators such as fish and birds utilise intertidal rocky reef habitats The ecology and importance of species that are becoming targets of food and bait collection, but which have not traditionally been collected in Victoria Interactions between coastal oceanography and intertidal communities, i.e. effects of upwelling such as on the Bonney coast How water level rises will affect reef assemblages - e.g. are there low lying reefs that will be completely covered? Subtidal reefs Plummer et al. (2003) summarised the habitat types and species found on shallow reefs within marine national parks and sanctuaries in Victoria. For many sites, they noted that while anecdotal or qualitative data existed, quantitative data were often lacking. It has been suggested that on shallow reefs in Victoria, surrogates such as dominant vegetation and region may be more accurate for predicting assemblage structure, at least for algae and invertebrates (O'Hara 2001), than substrate alone, and indeed recent predictive models appear to be satisfactory for linking substrate and depth with macroalgae (Holmes et al. 2008). Determining sources of larval recruitment and causes of variability in recruitment Standardising commercial catch rates and determining stock status Gaps in our knowledge about shallow reefs, including those in Port Phillip Bay and along Victoria’s open coastline include: Investigating the effect of seawater temperature of rock lobster ecology including the effect of the Bonny upwelling nutrient rich intrusion into western Victoria. Basic information on what species are found and assemblage structure - Algae - Sessile invertebrates - Mobile invertebrates - Fish Quantitative data examining patterns of spatial and temporal variation Relationships with assemblages from adjacent habitats, e.g. sand and seagrass patches The impact of introduced species Relationship with coastal oceanography such as the Bonney upwelling Processes driving the ecology of sheltered subtidal reefs in Port Phillip Bay compared to exposed reefs on the open coast Very little research has been carried out on invertebrates on subtidal reefs of Port Phillip Bay Processes relating to the export of drift algae and detritus from subtidal reefs and the subsidy to other habitats (Vanderklift and Wernberg 2008; Crawley et al. 2009). Key knowledge gaps for commercial fisheries of abalone and rock lobster exist and have been identified for the purposes of this literature review. Abalone research is required (Harry Gorfine pers. comm.) into: The quantification of stock-recruitment dynamics of the fishery Age validation of individual abalone specimens and Modelling the fishery as an entire system including management performance measures. Rock lobster research is required (David Reilly pers. comm.) into: Deep reefs and canyons Little is known about deep reefs in Victoria, or the biology and ecology of organisms that live on them, due in part to difficulties associated with conducting observational work or manipulative experiments in situ. Almost nothing is therefore known about how various biotic factors influence the distribution, survival, etc. of species found on deep reefs, except for those species such as rock lobster and abalone, where some information is available due to directed research on these valuable fisheries species (see previous discussion of these fisheries). Without basic information on the life history of organisms and details of how they interact with each other and the environment, it may not be possible to determine to what degree potential disturbance events have an impact on individual species or assemblages of species. Gaps in our knowledge about deep reefs, including those in Port Phillip Heads include: Basic information on what species are found and assemblage structure; this is true for both sessile species and associated mobile invertebrates Spatial and temporal patterns of distribution of species on deep reefs through Victoria Which species are ecologically important Rarity of species and assemblages in a wider geographical sense Processes determining the structure of communities in deep reefs and canyons, especially in Port Phillip Heads (current, sediment loads, plankton/seston concentration) Identification of species and assemblages that are vulnerable to natural and human disturbance/impacts The uniqueness of sessile invertebrate species in Port Phillip Heads deep reef and Canyon compared to other deep reef and canyon habitats in Victoria Temperate reefs literature review 35 Trophic linkages between deep reefs and canyon communities in Victoria and other marine and terrestrial communities. Due to this knowledge gap, key questions that we are currently unable to answer with confidence include: How the removal, increase or introduction of species may effect assemblage structure Temperate reefs literature review 36 How communities change on a temporal basis, whether that be seasonally or between years How assemblages may recover from pulse or press disturbance events How climatic or oceanographic changes may influence the distribution and health of assemblages. References Abdo, D. A., McDonald , J. I., Harvey, E. S., Fromont, J., and Kendrick, G. A. (2008). Neighbour and environmental influences on the growth patterns of two temperate Haliclonid sponges. Marine and Freshwater Research 59, 304-312. Addison, P. F. E., Koss, R. S., and O'Hara, T. D. (2008). Recreational use of a rocky intertidal reef in Victoria: implications for ecological research and management. Australasian Journal of Environmental Management 15, 169-179. Anderson, M. J., and Millar, R. B. (2004). Spatial variation and effects of habitat on temperate reef fish assemblages in northeastern New Zealand. Journal of Experimental Marine Biology and Ecology 305, 191-221. Anderson, T. J., Syms, C., Roberts, D. A., and Howard, D. F. (2009). Multi-scale fishhabitat associations and the use of habitat surrogates to predict the organisation and abundance of deepwater fish assemblages. Journal of Experimental Marine Biology and Ecology 379, 34-42. doi:10.1016/j.jembe.2009.07.033 Anderson, T. W. (1994). Role of macroalgal structure in the distribution and abundance of a temperate reef fish. Marine Ecology Progress Series 113, 279290. Andrew, N., and O'Neill, A. L. (2000). Largescale patterns in habitat structure on subtidal rocky reefs in New South Wales. Marine and Freshwater Research 51, 255-263. Andrew, N. L. (1993). Spatial heterogeneity, sea urchins grazing, and habitat structure on reefs in temperate Australia. Ecology 74, 292-302. Andrew, N. L. (Ed.) (1999). 'Under Southern Seas.' (University of New South Wales Press: Sydney.) Andrew, N. L., and MacDiarmid, A. B. (1991). Interrelations between sea urchins and spiny lobsters in northeastern New Zealand. Marine Ecology Progress Series 70, 211-222. Andrew, N. L., and Stocker, L. J. (1986). Dispersion and phagokinesis in the echinoid Evechinus chloroticus (Val.). Journal of Experimental Marine Biology and Ecology 100, 11-23. Andrew, N. L., and Underwood, A. J. (1992). Associations and abundance of sea urchins and abalone on shallow subtidal reefs in southern New South Wales. Australian Journal of Marine and Freshwater Research 43, 1547-1559. Australian Government (2005). Assessment of the Victorian Sea Urchin Fishery. Australian Government, Department of the Environment and Heritage. Axelrad, D. M., Poore, G. C. B., Arnott, G. H., Bauld, J., Brown, V. E., R.R.C., and Hickman, N. J. (1981). The effects of treated sewage discharge on the biota of Port Phillip Bay, Victoria, Australia. In 'Estuaries and Nutrients'. (Eds B. J. Neilson and L. G. Cronin.). (The Humana Press) Ayling, A. M. (1981). The role of biological disturbance in temperate subtidal encrusting communities. Ecology 62, 830847. Baines, P. G., and Fandry, C. B. (1983). Annual cycle of the density field in Bass Strait. Australian Journal of Marine and Freshwater Research 34, 143-153. Baker, K. (2006). Top-down and Bottom-up regulation of rocky intertidal shores of southeastern Victoria, Australia. Honours Thesis, University of Melbourne. Ball, D., and Blake, S. (2007a). Shallow water habitat mapping at Victorian Marine National Parks and Marine Sanctuaries, Volume 1: Western Victoria. Parks Victoria Technical Series No.36. Parks Victoria, Melbourne. Ball, D., and Blake, S. (2007b). Shallow water habitat mapping at Victorian Marine National Parks and Marine Sanctuaries, Volume 2: Eastern Victoria. Parks Victoria Technical Series No.37. Parks Victoria, Melbourne. Temperate reefs literature review 37 Ball, D., Blake, S., and Plummer, A. (2006). Review of Marine Habitat Classification Systems No. 26. Parks Victoria, Melbourne. Ball, D., Morris, L., Womersley, B., Blake, S., and Coots, A. (In prep). Habitat assessment of rock lobster fixed site survey areas. Department of primary Industries, Queenscliff, Victoria. Bax, N. J., and Williams, A. (2001). Seabed habitat on the south-eastern Australian continental shelf: context, vulnerability and monitoring. Marine and Freshwater Research 52, 491-512. Bax, N. J., and Williams, A. E. (2000). Habitat and fisheries productivity in the South East Fishery. Final report to FRDC Project 94/040. Marine Research, Hobart, Tasmania, Australia. Beaman, R. J., Daniell, J. J., and Harris, P. T. (2005). Geology-benthos relationships on a temperate rocky bank, eastern Bass Strait, Australia. Marine and Freshwater Research 56, 943-958. Bell, J. D., Burchmore, J. J., and Pollard, D. A. (1978). Feeding ecology of three sympatric species of leatherjackets (Pisces: Monacanthidae) from a Posidonia seagrass habitat in New South Wales. Australian Journal of Marine and Freshwater Research 29, 631-643. Bell, J. J., and Barnes, D. K. A. (2000). The influences of bathymetry and flow regime upon the morphology of sublittoral sponge communities. Journal of the Marine Biological Association of the United Kingdom 80, 707-718. Bell, J. J., Barnes, D. K. A., and Turner, J. R. (2002). The importance of micro and macro morphological variation in the adaptation of a sublittoral demosponge to current extremes. Marine Biology 140, 75-81. Bellgrove, A. (1998). Recruitment of intertidal macroalgae on a wave exposed rocky coast. PhD Thesis, Monash University. Bellgrove, A., Clayton, M. N., and Quinn, G. P. (1997). Effects of secondarily treated sewage effluent on intertidal macroalgal recruitment processes. Marine and Freshwater Research 48, 137-146. Bellgrove, A., Clayton, M. N., and Quinn, G. P. (2004). An integrated study of the temporal and spatial variation in the Temperate reefs literature review 38 supply of propagules, recruitment and assemblages of intertidal macroalgae on a wave-exposed rocky coast, Victoria, Australia. Journal of Experimental Marine Biology and Ecology 310, 207-225. doi:10.1016/j.jembe.2004.04.011 Benedetti-Cecchi, L., Pannacciulli, F., Bulleri, F., Moschella, P. S., Airoldi, L., Relini, G., and Cinelli, F. (2001). Predicting the consequences of anthropogenic disturbance: large scale effects of loss of canopy algae on rocky shores. Marine Ecology Progress Series 214, 137-150. Bennett, I., and Pope, E. C. (1952). Intertidal zonation of the exposed rocky shores of Victoria, together with a rearrangement of the biogeographical provinces of temperate Australian shores. Australian Journal of Marine and Freshwater Research 4, 105-159. Bennett, M. (1990). The effect of grazing gastropods on the recovery of a disturbance within the Hormosira mat. Honours Thesis, University of Melbourne. Beovich, E. K., and Quinn, G. P. (1992). The grazing effects of limpets on a rocky intertidal shore. Australian Journal of Ecology 17, 75-82. Black, K. P. (1992). Evidence of the importance of deposition and winnowing of surficial sediments at a continental shelf scale. . Journal of Coastal Research 8, 319331. Black, K. P., Hatton, D., and Rosenberg, M. (1993). Locally and externally-driven dynamics of a large semi-enclosed bay in southern Australia. Journal of Coastal Research 9, 509-538. Blake, S., Young, P., Ball, D., and Coots, A. (2009a). Corangamite Nearshore Marine Habitat Mapping and Assessment, Fisheries Victoria Technical Report Series. Department of Primary Industries, Queenscliff, Victoria, Australia. Blake, S., Young, P., Ball, D., and Coots, A. (2009b). Nearshore Marine Habitat Mapping and Assessment. Fisheries Victoria, Department of Primary Industries, Queenscliff, Victoria, Australia. Bodkin, J. L. (1988). Effects of kelp forest removal on associated fish assemblages in central California. Journal of Experimental Marine Biology and Ecology 117, 227-238. Bokn, T. L., Duarte, C. M., Pedersen, M. F., Marba, N., Moy, F. E., Barrón, C., Bjerkeng, B., Borum, J., Christie, H., Engelbert, S., Fotel, F. L., Hoell, E. E., Karez, R., Kersting, K., Kraufvelin, P., Lindblad, C., Olsen, M., Sanderud, K. A., Sommer, U., and Sørensen, K. (2003). The response of experimental rocky shore communities to nutrient additions. Ecosystems 6, 577-594. Braley, H., Anderson, T. A., and Quinn, G. P. (1991). The effect of the grazing gastropod Bembicium nanum on recolonization of algae on an intertidal rock platform. Proceedings of the Royal Society of Victoria 103, 13-16. Breaker, L. C., and Broenkow, W. W. (1994). The circulation of monterey bay and related processes. In 'Oceanography and Marine Biology, Vol 32' pp. 1-64) Brown, V. B., Davies, S. A., and Synnot, R. N. (1990). Long-term monitoring of the effects of treated sewage effluent on the intertidal macroalgal community near Cape Schanck, Victoria, Australia. Botanica Marina 33, 85-98. Brown, V. B., Rowan, K. S., and Ducker, S. C. (1980). The Effects of Sewage Effluent on the Macrophytes off Werribee, Port Phillip Bay. Ministry of Conservation Victoria, Environmental Studies Program, Task Report No. 273. Bryan, G. W., Gibbs, P. E., Burt, G. R., and Hummerstone, L. G. (1987). The effects of tributyltin (TBT) accumulation on adult dogwhelks, Nucella lapillus; long term field and laboratory experiments. Journal of the Marine Biological Association of the United Kingdom 67, 525-544. Burn, R. (2006). A checklist and bibliography of the Opisthobranchia (Mollusca: Gastropoda) of Victoria and the Bass Strait area, south-eastern Australia. Museum Victoria Science Reports 10, 1106. Burton, B. (1999). Competitive interactions between and within the intertidal gastropod genera Cellana and Siphonaria. PhD Thesis, University of Melbourne. Butler, A., Althaus, F., Furlani, D., and Ridgway, K. (2002a). Assessment of the conservation values of the Bass Strait sponge beds area: a component of the Commonwealth Marine Conservation Assessment Program 2002-2004: report to Environment Australia. Butler, A., Althaus, F., Furlani, D., and Ridgway, K. (2002b). Assessment of the conservation values of the Bonney upwelling area: a component of the Commonwealth Marine Conservation Assessment Program 2002-2004 : report to Environment Australia CSIRO, Hobart, Tasmania, Australia. Campbell, S. J. (1999). Occurrence of Codium fragile subsp Tomentosoides (Chlorophyta : Bryopsidales) in marine embayments of southeastern Australia. Journal of Phycology 35, 938-940. Carballo, J. L., and Nava, H. (2007). A comparison of sponge assemblage patterns in two adjacent rocky habitats (tropical Pacific Ocean, Mexico). Écoscience Carey, J. M., Beilin, R., Boxshall, A., Burgman, M. A., and Flander, L. (2007). Risk-Based Approaches to Deal with Uncertainty in a Data-Poor System: Stakeholder Involvement in Hazard Identification for Marine National Parks and Marine Sanctuaries in Victoria, Australia. Risk Analysis 27, 271-281. Carr, M. H. (1989). Effects of macroalgal assemblages on the recruitment of temperate zone reef fishes. Journal of Experimental Marine Biology and Ecology 126, 59-76. Cheshire, A. C., and Hallum, N. D. (1989). Biomass and density of native stands of Durvillaea potatorum (southern bull-kelp) in south eastern Australia. Marine Ecology Progress Series 8, 277-283. Chidgey, S. (2006). Basslink Project Marine Biological Monitoring: McGaurans Beach. CEE Consultants Pty Ltd, Richmond, Victoria, Australia. Chidgey, S. S., and Marshall, P. A. (1994). Progress report on ground-truthing survey, June 1994, Port Phillip Bay Environmental Study. Choat, J. H., and Ayling, A. M. (1987). The relationship between habitat structure and fish faunas on New Zealand reefs. Journal of Experimental Marine Biology and Ecology 110, 257-284. Temperate reefs literature review 39 Choat, J. H., and Schiel, D. R. (1982). Patterns of distribution and abundance of large brown algae and invertebrate herbivores in subtidal regions of northern New Zealand. Journal of Experimental Marine Biology and Ecology 60, 129-162. Cirano, M., and Middleton, J. F. (2004). Aspects of the mean wintertime circulation along Australia's southern shelves. Journal of Physical Oceanography 34, 668684. Clayton, M. N. (1990). The adaptive significance of life history characters in selected orders of marine brown macroalgae. Australian Journal of Ecology 15, 439-452. Coleman, M. A., Vytopil, E., Goodsell, P. J., Gillanders, B. M., and Connell, S. D. (2007). Diversity and depth-related patterns of mobile invertebrates associated with kelp forests. Marine and Freshwater Research 58, 589-595. Coleman, R. F. (1972). Observations on shallow water, rocky reef fishes of port Phillip Bay, Victoria. B.Sc. Thesis. Connell, S. D. (2003a). The monopolization of understorey habitat by subtidal encrusting coralline algae: a test of the combined effects of canopy-mediated light and sedimentation. Marine Biology 142, 1065-1071. Connell, S. D. (2003b). Negative effects overpower the positive of kelp to exclude invertebrates from the understorey community. Oecologia 137, 97-103. Connell, S. D. (2005). Assembly and maintenance of subtidal habitat heterogeneity: synergistic effects of light penetration and sedimentation. Marine Ecology Progress Series 289, 53-61. Connell, S. D. (2007). Subtidal temperate rocky habitats: Habitat heterogeneity at local to continental scales. In 'Marine Ecology'. (Eds S. D. Connell and B. M. Gillanders.) pp. 378-401. (Oxford University Press: South Melbourne.) Connell, S. D., and Gillanders, B. M. (2007). 'Marine Ecology.' (Oxford University Press: Oxford.) Connell, S. D., and Irving, A. D. (2008). Integrating ecology with biogeography using landscape characteristics: a case study of subtidal habitat across Temperate reefs literature review 40 continental Australia. Journal of Biogeography 35, 1608-1621. Connell, S. D., and Irving, A. D. (2009). The subtidal ecology of rocky-coasts: localregional-biogeographic patterns and their experimental analysis. In 'Marine macroecology'. (Eds J. D. Witman and R. Kaustuv.) pp. 392-417. (University of Chicago Press: Chicago.) Crawley, K. R., Hyndes, G. A., Vanderklift, M. A., Revill, A. T., and Nichols, P. D. (2009). Allochthonous brown algae are the primary food source for consumers in a temperate, coastal environment. Marine Ecology Progress Series 376, 33-44. doi:10.3354/meps07810 Creese, R. G., and Underwood, A. J. (1982). Analysis of inter- and intra- specific competition amongst intertidal limpets with different methods of feeding. Oecologia 53, 337-346. Curdia, J., Carvalho, S., Ravara, A., Gage, J. D., Rodrigues, A. M., and Quintino, V. (2004). Deep macrobenthic communities from Nazare submarine canyon (NW Portugal). ' pp. 171-180. (Inst Ciencias Mar Barcelona.) Curley, B. G., Kingsford, M. J., and Gillanders, B. M. (2002). Spatial and habitat-related patterns of temperate reef fish assemblages: implications for the design of Marine Protected Areas. Marine and Freshwater Research 53, 1197-1210. Daily, G. C., Alexander, S., Ehrlich, P. R., Goulder, L., Lubchenco, J., Matson, P. A., Mooney, H. A., Postel, S., Schneider, S. H., Tilman, D., and Woodwell, G. M. (1997). Ecosystem Services: Benefits Supplied to Human Societies By Natural Ecosystems. Issues in Ecology 2, 1-16. Dartnall, A. J. (1974). Littoral biogeography. In 'Biogeography and Ecology in Tasmania'. (Ed. W. D. Williams.) pp. 171-194. (Dr. W. Junk b.v., Publishers: The Hague.) Daume, S., Brand-Gardner, S., and Woelkerling, W. J. (1999). Preferential settlement of abalone larvae: diatom films vs. nongeniculate coralline red algae. Aquaculture 174, 243-254. Day, R., Dowell, A., Sant, G., Klemke, J., and Shaw, C. (1995). Patchy predation: Foraging behaviour of Coscinasterias calamaria and escape responses for Haliotis rubra. Marine and Freshwater Behaviour and Physiology 26, 11-33. Dayton, P. K., Currie, V., Gerrodette, T., Keller, B. D., Rosenthal, R., and VenTresca, D. (1984). Patch dynamics and stability of some California kelp communities. Ecological Monographs 54, 253-289. Department of Primary Industries (2009a). 'The Victorian Abalone Fishery.' Available at http://www.dpi.vic.gov.au/DPI/nrenfaq. nsf/LinkView/9F09A1B4418E935BCA256 C55001EB9A9F5F3C3DA915AFBE4CA2 56C6F0016CA60 [accessed 11/11/2009. Department of Primary Industries (2009b). Victorian Rock Lobster Fishery Management Plan. Fisheries Victoria Management Report Series No. 70. Melbourne. Department of the Environment and Heritage (2005). Assessment of the Victorian Sea Urchin Fishery. Australian Government, Canberra. Department of the Environment, W., Heritage and the Arts (2008). 'National Representative System of Marine Protected Areas (NRSMPA).' Available at http://www.environment.gov.au/coasts/ mpa/nrsmpa/index.html. Dixon, C. D., Gorfine, H. K., Officer, R. A., and Sporcic, M. (1998). Dispersal of tagged blacklip abalone, Haliotis rubra: Implications for stock assessment. Journal of Shellfish Research 17, 881-887. Doblin, M., and Clayton, M. N. (1995). The effects of secondarily treated sewage effluent on the early life history stages of two species of brown macroalgae: Hormosira banksii and Durvillea potatorum. Marine Biology 122, 689-698. Duckworth, A. R. (2003). Effect of wound size on the growth and regeneration of two temperate subtidal sponges. Journal of Experimental Marine Biology and Ecology 287, 139-153. Duggins, D. O. (1989). Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245, 170-173. Edgar, G. J. (1984). General features of the ecology and biogeography of tasmanian subtidal rocky shore communities. Papers and Proceedings of the Royal Society of Tasmania 118, 173-186. Edmunds, M., Hart, S., Elias, J., and Jenkins, S. (2003). Victorian subtidal reef monitoring program: The reef biota at Port Phillip Heads Marine National Park. Parks Victoria, Melbourne. Edmunds, M., Hart, S., Elias, J., and Power, B. (2004). Victorian intertidal reef monitoring program: The reef biota in Central Victoria and Port Phillip Bay Marine Sanctuaries. Parks Victoria, Melbourne. Edmunds, M., Judd, A., Gilmour, P., Stewart, K., Pickett, P., Monk, J., and Crozier, J. (2006a). Volume 8: Shallow Reefs. Report to Maunsell. Australian Marine Ecology Report 356, Melbourne. Edmunds, M., Power, B., Pickett, P., Baker, K., Wassnig, M., Crozier, J., Monk, J., Gilmour, P., Shimeta, J., Sams, M., Judd, A., Williams, J., and Stewart, K. (2006b). Volume 9: Deep Reef Biota. Report to Maunsell. Australian Marine Ecology Report 357, Melbourne. Edmunds, M., Roob, R., and Ferns, L. W. (2000). Chapter 6 Marine biogeography of central Victoria and Flinders bioregions - A preliminary analysis of reef flora and fauna. In 'Environmental inventory of Victoria's Marine Ecosystems Stage 3 (2nd Edition) - Understanding Biodiversity Representativeness of Victoria's Rocky Reefs'. (Eds L. W. Ferns and D. Hough.). (Parks, Flora and Fauna Division, Department of Natural Resources and Environment: East Melbourne.) Elias, J., Edmunds, M., and Hart, S. (2004). Port Phillip Bay channel deepening environmental effects statement marine ecology specialist studies. Volume 7: deep reef habitat study. Port of Melbourne Corporation, Melbourne. Environment Conservation Council (2000). Marine Coastal & Estuarine Investigation Final Report. Environment Conservation Council, East Melbourne. Fairweather, P. G. (1990). Sewage and the biota on seashores: assessment of impact in relation to natural variability. Environmental Maonitoring and Assessment 14, 197-210. Fairweather, P. G. (1991). A conceptual framework for ecological studies of coastal resources: an example of a Temperate reefs literature review 41 tunicate collected for bait on Australian seashores. Ocean and shoreline management 15, 125-142. Ferns, L. (2003). Victoria’s system of marine national parks and marine sanctuaries: management strategy 2003-2010. Melbourne, Australia. Ferns, L. W., and Hough, D. (2000). Environmental inventory of Victoria's Marine Ecosystems Stage 3 (2nd Edition). Parks, Flora and Fauna Division, Department of Natural Resources and Environment, East Melbourne. Ferns, L. W., Hough, D., and Caitlin, J. (2000). Chapter 1 Synthesis of stage 3. Describing marine biodiversity through mapping and quantitative analysis of biological data: A classification system for Victoria's intertidal and nearshore sub-tidal marine waters. In 'Environmental inventory of Victoria's Marine Ecosystems Stage 3 (2nd Edition) - Understanding Biodiversity Representativeness of Victoria's Rocky Reefs'. (Eds L. W. Ferns and D. Hough.). (Parks, Flora and Fauna Division, Department of Natural Resources and Environment: East Melbourne.) Fletcher, H., and Frid, C. L. J. (1996). Impact and management of visitor pressure on rocky intertidal algal communities. Aquatic conservation: Marine and freshwater ecosystems 6, 287-297. Flora and fauna guarantee - scientific advisory committee (2009). Final recommendation on a nomination for listing: Port Phillip Bay entrance deep canyon marine community. Nomination no. 794. Foale, S. (1993). An evaluation of the potential of gastropod imposex as a bioindicator of tributyltin pollution in Port-Phillip Bay, Victoria. Marine Pollution Bulletin 26, 546-552. Fulton, C. J., and Bellwood, D. R. (2004). Wave exposure, swimming performance, and the structure of tropical and temperate reef fish assemblages. Marine Biology 144, 429-437. Garcı´a-Charton, J. A., and Pe´rez-Ruzafa, A. (2001). Spatial pattern and the habitat structure of a Mediterranean rocky reef Temperate reefs literature review 42 fish local assemblage. Marine Biology 138, 917-934. Gibbs, P. E., Bryan, G. W., Pascoe, P. L., and G.R., B. (1987). The use of the dogwhelk, Nucella lapillus as an indicator of tributyltin (TBT) contamination. Journal of the Marine Biological Association of the United Kingdom 67, 507-523. Gill, E. D., Segnit, E. R., and Hunt, I. (1980). Pleistocene submerged cliff off the Otway coast of Victoria. Proceeding of the Royal Society of Victoria 91, 43-51. Gillanders, B. M. (1995). Feeding ecology of the temperate marine fish Achoerodus viridis (Labridae): size, seasonal and sitespecific differences. Marine and Freshwater Research 46, 1009-1020. Gillanders, B. M. (1997a). Comparison of growth rates between estuarine and coastal reef populations of Achoerodus viridis (Pisces: Labridae). Marine Ecology Progress Series 146, 283-287. Gillanders, B. M. (1997b). Patterns of abundance and size structure in the blue groper, Achoerodus viridis (Pisces, Labridae): evidence of links between estuaries and coastal reefs. Environmental Biology of Fishes 49, 153-173. Gillanders, B. M., and Kingsford, M. J. (1998). Influence of habitat on abundance and size structure of a large temperate reef fish, Achoerodus viridis (Pisces : Labridae). Marine Biology 132, 503-514. Gilmour, P., and Edmunds, M. (2007). Victorian intertidal reef monitoring program: The intertidal reef biota of Central Victoria's Marine Protected Areas (Volume 2). Parks Victoria, Melbourne. Goodsell, P. J., Fowler-Walker, M. J., Gillanders, B. M., and Connell, S. D. (2004). Variations in the configuration of algae in subtidal forests: Implications for invertebrate assemblages. Austral Ecology 29, 350-357. Gorgula, S. C., S., (2004). Expansive covers of turf-forming algae on human dominated coast: the relative effects of increasing nutrient and sediment loads. Marine Biology 145, 613-619. Harris, P. T. (2007). Applications of geophysical information to the design of a representative system of marine protected areas in southeastern Australia. In 'Mapping the seafloor for habitat characterisation: Geological Association of Canada special paper 47'. (Eds B. J. Todd and G. Greene.) pp. 449468: St Johns, NF, Canada.) Harris, P. T., Heap, A. D., Anderson, T. J., and Brooke, B. (2009). Comment on: Williams et al. (2009) "Australia's deepwater reserve network: implications of false homogeneity for classifying abiotic surrogates of biodiversity" ICES Journal of Marine Science, 66: 214–224. ICES Journal of Marine Science 2082-2085 Harris, P. T., Heap, A. D., Passlow, V., Sbaffi, L., Fellows, M., Porter-Smith, R., Buchanan, C., and Daniell, J. (2005). Geomorphic features of the continental margin of Australia: report to the National Oceans Office on the production of a consistent, high-quality bathymetric data grid and definition and description of geomorphic units for part of Australia’s marine jurisdiction. Geoscience Australia, Canberra. Hawkins, S. J. (1981). The influence of season and barnacles on the algal colonization of Patella vulgata exclusion areas. Journal of the Marine Biological Association of the United Kingdom 61, 1-15. Hidas, E. Z., Costa, T. L., Ayre, D. J., and Minchinton, T. E. (2007). Is the species composition of rocky intertidal invertebrates across a biogeographic barrier in south-eastern Australia related to their potential for dispersal? Marine and Freshwater Research 58, 835842. Hill, M. S., and Hill, A. L. (2002). Morphological plasticity in the tropical sponge Anthosigmella varians: responses to predators and wave energy. The Biological Bulletin 202, 86-95. Hindell, J. S., and Quinn, G. P. (2000). Effects of sewage effluent on the population structure of Brachidontes rostratus (Mytilidae) on a temperate intertidal rocky shore. Marine and Freshwater Research 51, 543-551. Holbrook, S. J., Carr, M. H., Schmitt, R. J., and Coyer, J. A. (1990a). Effect of giant kelp on local abundance of reef fishes: the importance of ontogenetic resource requirements. Bulletin of Marine Science 47, 104-114. Holbrook, S. J., Kingsford, M. J., Schmitt, R. J., and Stephens Jr, J. S. (1994). Spatial and temporal patterns in assemblages of temperate reef fish. American Zoologist 34, 463-475. Holbrook, S. J., Schmitt, R. J., and Ambrose, R. F. (1990b). Biogenic habitat structure and characteristics of temperate reef fish assemblages. Australian Journal of Ecology 15, 489-503. Holmes, K. W., Radford, B., Van Niel, K. P., Kendrick, G. A., Grove, S. L., and Chatfield, B. (2007). Mapping the benthos in Victoria’s Marine National Parks, 1. Cape Howe Marine National Park. Parks Victoria, Melbourne. Holmes, K. W., Van Niel, K. P., Radford, B., Kendrick, G. A., and Grove, S. L. (2008). Modelling distribution of marine benthos from hydroacoustics and underwater video. Continental Shelf Research 28, 1800-1810. Hope Black, J. (1971). Benthic communities. Memoirs of The National Museum of Victoria Melbourne 32, 129-170. Hume, T. M., Oldman, J. W., and Black, K. P. (2000). Sediment facies and pathways of sand transport about a large deep water headland, Cape Rodney, New Zealand. New Zealand Journal of Marine and Freshwater Research 34, 695-717. Hunt, T. L. (2007). Ecological determinants of recruitment in populations of the southern hulafish, Trachinops caudimaculatus. Bachelor of Science, Degree with Honours Thesis Honours Thesis Thesis, University of Melbourne. IMCRA (1998). Interim Marine and Coastal Regionalisation for Australia: am ecosystem-based classification for marine and coastal environments. Version 3.3. Environment Australia, Commonwealth Department of Environment, Canberra, Australia. Irving, A. D., Connell, S. D., and Gillanders, B. M. (2004). Local complexity in patterns of canopy-benthos associations produces regional patterns across temperate Australasia. Marine Biology 144, 361-368. Jackson, J. B. C. (1986). Modes of dispersal of clonal benthic invertebrates: consequences for species distributions and genetic structure of local Temperate reefs literature review 43 populations. Bulletin of Marine Science 39, 588-606. Jenkins, G. P. (2004). The ecosystem effects of abalone fishing: a review. Marine and Freshwater Research 55, 545-552. Jenkins, G. P., Gason, A. S. H., and Morris, L. (2005a). Towards Ecosystem Based Management of the Victorian Abalone Fishery: Analysis of Existing Ecological Monitoring Data. Department of Primary Industries, Victoria, Queenscliff. Jenkins, G. P., Morris, L. C., and Blake, S. (2005b). Ecological Risk Assessment of the Victorian Rock Lobster Fishery. Department of Primary Industries, Victoria, Queenscliff. Jenkins, G. P., Watson, G. F., Hammond, L. S., Black, K. P., Wheatley, M. J., and Shaw, C. (1996). Importance of shallow water, reef-algal habitats as nursery areas for commercial fish from southeastern Australia. Fisheries Research and Development Corporation, 92/44. Jenkins, G. P., and Wheatley, M. J. (1998). The influence of habitat structure on nearshore fish assemblages in a southern Australian embayment: Comparison of shallow seagrass, reefalgal and unvegetated sand habitats, with emphasis on their importance to recruitment. Journal of Experimental Marine Biology and Ecology 221, 147-172. Jennings, J. N. (1958). The submarine topography of Bass Strait. Proceeding of the Royal Society of Victoria 71, 49-71. Jones, G. P. (1984). Population ecology of the temperate reef fish Pseudolabrus celidotus Bloch and Schneider (Pisces: Labridae). I. Factors influencing recruitment. Journal of Experimental Marine Biology and Ecology 75, 257-276. Jones, G. P., and Andrew, N. L. (1990). Herbivory and patch dynamics on rocky reefs in temperate Australasia: The roles of fish and sea urchins. Australian Journal of Ecology 15, 505-520. Jones, G. P., and Norman, M. D. (1986). Feeding selectivity in relation to territory size in a herbivorous reef fish. Oecologia 68, 549556. Kaandorp, J. A. (1999). Morphological analysis of growth forms of branching sessile Temperate reefs literature review 44 organisms along environmental gradients. Marine Biology 134, 295-306. Kaandorp, J. A., and Kluijver, M. J. d. (1992). Verification of fractal growth models of the sponge Haliclona oculata (Porifera) with transplantation experiments. Marine Biology 113, 133-143. Kaehler, S., and Williams, G. A. (1997). Do factors influencing recruitment ultimately determine the distribution and abundance of encrusting algae on seasonal tropical shores? Marine Ecology Progress Series 156, 87-96. Keough, M. J. (1988). Benthic Populations: is recruitment limiting or just fashionable? In 'Proceedings of the 6th International Coral Reefs Symposium '. pp. 141-148. Keough, M. J. (1999). Sessile Animals. In 'Under Southern Seas, the ecology of Australia's Rocky Reefs'. (Ed. N. Andrew.). (UNSW: Sydney.) Keough, M. J., Black, K. P., Russell, J. S., and Craig, W. O. (1996). Predicting the Scale of Marine Impacts: Understanding Planktonic Links between Populations. In pp. 199-234. (Academic Press: San Diego.) Keough, M. J., and Butler, A. J. (1996). Chapter 11. Temperate reefs. In 'The state of the marine environment report for Australia'. (Ed. L. P. Zann.). (GBRMPA: Townsville.) Keough, M. J., and King, A. (1991). Recommendations for monitoring of marine plant and animal populations in Wilsons Promontory Marine National Park and the Bunurong Marine Park. Melbourne. Keough, M. J., and Quinn, G. P. (1998). Effects of periodic disturbances from trampling on rocky intertidal algal beds. Ecological Applications 8, 141-161. Keough, M. J., and Quinn, G. P. (2000). Legislative vs. practical protection of an intertidal shoreline in southeastern Australia. Ecological Applications 10, 871881. Keough, M. J., Quinn, G. P., and Bathgate, R. (1997). Geographic variation in interactions between size classes of the limpet Cellana tramoserica. 215, 19-34. Keough, M. J., Quinn, G. P., and King, A. (1990). The ecology of temperate reefs. Australian Journal of Ecology 15, 361-363. Keough, M. J., Quinn, G. P., and King, A. (1993). Correlations between human collecting and intertidal mollusk populations on rocky shores. Conservation Biology 7, 378390. Keough, M. J., and Swearer, S. E. (2007). Fundamental concepts in marine ecology. In 'Marine Ecology'. (Eds S. D. Connell and B. M. Gillanders.) pp. 17-46. (Oxford University Press: South Melbourne.) King, A. (1992). Human activity and its effects on marine intertidal plant and animal populations: monitoring and management. B.Sc. Thesis, University of Melbourne. King, R. J., Hope Black, J., and Ducker, S. C. (1971). Intertidal ecology of Port Phillip Bay with systematic list of plants and animals. Memoirs of The National Museum of Victoria Melbourne 32, 93-128. Kloser, R. J., Williams, A., and Butler, A. (2001). Assessment of acoustic mapping of seabed habitats: marine biological and resource surveys South-East Region. Cooperative Program, Report 2 to the National Oceans Office. Land Conservation Council (1993). Marine and coastal special investigation, Descriptive report, June 1993. Larcombe, J., Brooks, K., Charalambou, C., Fenton, M., Fisher, M., Kinloch, M., and Summerson, R. (2002). Marine MattersAtlas of Marine Activities and Coastal Communities in Australia’s South-East Marine Region. Bureau of Rural Sciences, Canberra. Levin, L. A., Etter, R. J., Rex, M. A., Gooday, A. J., Smith, C. R., Pineda, J., Stuart, C. T., Hessler, R. R., and Pawson, D. (2001). Environmental influences on regional deep-sea species diversity. Annual Review of Ecology and Systematics 32, 5193. Lewis, J. A. (1975). The biology of Grateloupia filicina (Lamouroux) C. Agardh in Port Phillip Bay, Victoria. BSc Thesis, University of Melbourne. Lewis, J. A. (1983). Floristic composition and periodicity of subtidal algae on an artificial structure in port Phillip Bay (Victoria, Australia). Aquatic Botany 15, 257-274. Lewis, J. R. (1964). 'Ecology of rocky shores.' (The English Universities Press: London.) Light, B. R., and Woelkerling, W. J. (1992). 'Literature and information review of the benthic flora of Port Phillip Bay, Victoria, Australia / B.R. Light and Wm. J. Woelkerling.' (CSIRO: Melbourne :.) Little, C., Williams, G. A., and Trowbridge, C. (2009). 'The Biology of Rocky Shores.' (Oxford University Press: Oxford.) Littler, M. M., and Murray, S. N. (1975). Impact of sewage on the distribution, abundance and community structure of rocky intertidal macro-organisms. Marine Biology 30, 277-291. Love, M., Yoklavich, M., and Schroeder, D. (2009). Demersal fish assemblages in the Southern California Bight based on visual surveys in deep water. Environmental Biology of Fishes 84, 55-68. doi:10.1007/s10641-008-9389-8 Maldonado, M., and Young, C. M. (1996). Bathymetric patterns of sponge distribution on the Bahamian slope. Deep-Sea Research Part I-Oceanographic Research Papers 43, 897-915. Marshall, D. (2002). In situ measures of spawning synchrony and fertilization success in an intertidal free spawning invertebrate. Marine Ecology Progress Series 236, 113-119. Marshall, P. A., and Keough, M. J. (1994). Asymmetry in intraspecific competition in the limpet Cellana tramoserica (Sowerby). Journal of Experimental Marine Biology and Ecology 177, 121-138. McKenzie, P., and Bellgrove, A. (2008). Dispersal of Hormosira banksii (phaeophycae) via detached fragments: reproductive viability and longevity. Journal of Phycology 44, 1108-1115. McKenzie, P. F., and Bellgrove, A. (2006). No outbreeding depression at a regional scale for a habitat-forming intertidal alga with limited dispersal. Marine and Freshwater Research 57, 655-663. doi:10.1071/mf05078 McShane, P., and Smith, M. (1986). Starfish vs abalone in Port Philip bay. Australian Fisheries 148, 16-18. McShane, P. E. (1999). Blacklip Abalone. In 'Under Southern Seas, the ecology of Temperate reefs literature review 45 Australia's Rocky Reefs'. (Ed. N. Andrew.). (UNSW: Sydney.) McShane, P. E., Beinssen, K. H. H., and Foley, S. (1986). Abalone reefs in Victoria - a resource atlas. Marine Science Laboratories, Queenscliff. Menge, B. A. (2000). Top-down and bottom-up community regulation in marine rocky intertidal habitats. Journal of Experimental Marine Biology and Ecology 250, 257-289. Merory, M. (1997). Some influences on the feeding rate of the limpet Cellana tramoserica. Honours Thesis, University of Melbourne. Mettam, C. (1994). Intertidal zonation of animals and plants on rocky shores in the Bristol Channel and Severn Estuary-the northern shores. Biological Journal ofthe Linnean Society 51, 123-147. Middleton, J. F., and Black, K. P. (1994). The low frequency circulation in and around Bass Strait: a numerical study. Continental Shelf Research Monk, J., Ierodiaconou, D., Bellgrove, A., and Laurenson, L. (2008). Using Community-Based Monitoring with GIS to Create Habitat Maps for a Marine Protected Area in Australia. Journal of the Marine Biological Association of the United Kingdom 88, 865-871. Moore, C. H. (2008). Defining and predicting species-environment relationships: understanding the spatial ecology of demersal fish communities. PhD Thesis, The University of Western Australia. Moore, C. H., Harvey, E. S., and Van Niel, K. P. (2009). Spatial prediction of demersal fish distributions: enhancing our understanding of species-environment relationships. ICES Journal of Marine Science 66, 2068-2075. Nias, D. J., McKillup, S. C., and Edyvane, K. S. (1993). Imposex in Lepsiella vinosa from Southern Australia. Marine Pollution Bulletin 26, 380-384. Norman, M., and Jones, G. P. (1984). Determinants of territory size in the pomacentrid reef fish, Parma victoriae. Oecologia 61, 60-69. Nowell, A. R. M., and Jumars, P. A. J. (1984). Fluid environments of aquatic benthos. Annual Review of Ecology and Systematics 15, 303-328. Temperate reefs literature review 46 O'Brien, C. E. (1975). Standing crop, community composition and seasonal variations in two contrasting benthic algal communities in the Hobsons Bay area. BSc Thesis, University of Melbourne. O'Hara, T. (2000a). Chapter 3 Patterns of Temperate Marine Species Diversity at the Continental Scale. In 'Environmental inventory of Victoria's Marine Ecosystems Stage 3 (2nd Edition) Understanding Biodiversity Representativeness of Victoria's Rocky Reefs'. (Eds L. W. Ferns and D. Hough.). (Parks, Flora and Fauna Division, Department of Natural Resources and Environment: East Melbourne.) O'Hara, T. (2000b). Chapter 5 Habitats as surrogates for faunal and floral assemblages associated with rocky reef along the Victorian coast. In 'Environmental inventory of Victoria's Marine Ecosystems Stage 3 (2nd Edition) - Understanding Biodiversity Representativeness of Victoria's Rocky Reefs'. (Eds L. W. Ferns and D. Hough.). (Parks, Flora and Fauna Division, Department of Natural Resources and Environment: East Melbourne.) O'Hara, T. (2001). Consistency of faunal and floral assemblages within temperate subtidal rocky reef habitats. Marine and Freshwater Research 52, 853-863. O'Hara, T., McShane, P. E., and Norman, M. (1999). Victoria. In 'Under Southern Seas, the ecology of Australia's Rocky Reefs'. (Ed. N. Andrew.). (UNSW: Sydney.) O'Hara, T. D. (2002). Benthic assemblages of Bass Strait. Report to Geosciences Australia. Museum Victoria. O'Hara, T. D., Addison, P. F. E., Gazzard, R., Costa, T. L., and Pocklington, J. B. (In Press). A rapid bioassessment methodology tested on intertidal rocky shores. Aquatic Conservation O'Hara, T. D., and Poore, C. G. B. (2000). Patterns of distributions for southern Australian marine echinoderms and decapods. Journal of Biogeography 27, 1321-1335. Officer, R. A., Dixon, C. D., and Gorfine, H. K. (2001). Movement and re-aggregation of the blacklip abalone, Haliotis rubra Leach, after fishing. Journal of Shellfish Research 20, 771-779. Okey, T. A. (2003). Macrobenthic colonist guilds and renegades in Monterey Canyon (USA) drift algae: Partitioning multidimensions. Ecological Monographs 73, 415-440. Paine, R. T. (1966). Food web complexity and species diversity. The American Naturalist 100, 65-75. Paine, R. T. (1995). A conversation on refining the concept of keystone species. Conservation Biology 9, 962-964. Palumbi, S. R. (1984). Tactics of acclimation: morphological changes in sponges in an unpredictable environment. Science 225, 1478-1480. Palumbi, S. R. (1986). How body plans limit acclimation: responses of a demosponge to wave force. Ecology 67, 208-214. Parker Jr, R. O. (1990). Tagging Studies and Diver Observations of Fish Populations on Live-Bottom Reefs of the U.S. Southeastern Coast. Bulletin of Marine Science 46, 749-760. Parry, G. (1982). Reproductive effort in four species of intertidal limpets. Marine Biology 67, 267-282. Parry, G. D., and Restall, J. E. (2007). The effect of Boags Rocks sewage discharge on adjacent rocky reefs. Department of Primary Industries No. 64. Passlow, V., O’Hara, T. D., Daniell, T., Beaman, R. J., and Twyford, L. M. (2006). Sediments and benthic biota of Bass Strait: an approach to benthic habitat mapping. Plummer, A., L., M., Blake, S., and Ball, D. (2003). Marine natural values study: Victorian marine national parks and sanctuaries. Parks Victoria, Melbourne. Ponder, W., Hutchings, P., and Chapman, R. (2002). 'Overview of the conservation of Australian marine invertebrates. A report for Environment Australia.' Available at http://malsocaus.org/marine_invert/cont ents.html. Poore, G. C. B., McCallum, A. W., and Taylor, J. (2008). Decapod Crustacea of the continental margin of south-western and central Western Australia: preliminary identifications of 524 species from FRV Southern Surveyor voyage SS10-2005. Museum Victoria Science Reports 11, 1-106. Port of Melbourne Corporation (2007a). 13 - The bay - Project areas 2 and 3. Port of Melbourne Corporation, Melbourne. Port of Melbourne Corporation (2007b). 14 - The entrance - Project area 4. Port of Melbourne Corporation, Melbourne. Port of Melbourne Corporation (2007c). Appendix 51 - Overview Impact Assessment - Shallow Reef Communities. Port of Melbourne Corporation, Melbourne. Port of Melbourne Corporation (2007d). Appendix 52 - Overview Impact Assessment - Deep Canyon. Port of Melbourne Corporation, Melbourne. Povey, A., and Keough, M. J. (1991). Effects of trampling on plant and animal interactions on rocky shores. OIKOS 61, 355-368. Prince, J. D. (2003). The barefoot ecologist goes fishing. Fish and Fisheries 4, 359. Quinn, G. P. (1988). Effects of conspecific adults, macroalgae and height on the shore on recruitment of an intertidal limpet. Marine Ecology Progress Series 48, 305308. Quinn, G. P., and Ryan, N. R. (1989). Competitive interactions between two species of intertidal gastropod from Victoria, Australia. Journal of Experimental Marine Biology and Ecology 125, 1-12. Rattray, A., Ierodiaconou, D., Laurenson, L., Burq, S., and Reston, M. (2009). Hydroacoustic remote sensing of benthic biological communities on the shallow South East Australian continental shelf. Estuarine Coastal and Shelf Science 84, 237245. Rees, C. M., Brady, B. A., and Fabris, G. J. (2001). Incidence of imposex in Thais orbita from Port Phillip Bay (Victoria, Australia), following 10 years of regulation on use of TBT. Marine Pollution Bulletin 42, 873878. Rennie, S., Hanson, C. E., McCauley, R. D., Pattiaratchi, C., Burton, C., Bannister, J., Jenner, C., and Jenner, M. N. (2009). Physical properties and processes in the Perth Canyon, Western Australia: Links to water column production and seasonal pygmy blue whale abundance. Temperate reefs literature review 47 Journal of Marine Systems 77, 21-44. doi:10.1016/j.jmarsys.2008.11.008 Roberts, D., and Davis, A. (1996). Patterns in sponge assemblages on temperate coastal reefs off Sydney, Australia. Marine and Freshwater Research 47, 897906. Roberts, D. E. (1996). Patterns in subtidal marine assemblages associated with a deepwater sewage outfall. Marine and Freshwater Research 47, 1-9. Rollings, N., Light, B., Doblin, M., and Chiffings, A. (1993). An evaluation of remote sensing and associated field techniques for mapping the distribution of benthic habitats in Port Phillip Bay, Unpublished final report, Port Phillip Bay Environmental Study Task G2.1. Russell, B. C. (1983). The food and feeding habits of rocky reef fish of north-eastern New Zealand. New Zealand Journal of Marine and Freshwater Research 17, 121-145. Russell, B. D., and Connell, S. D. (2005). A novel interaction between nutrients and grazers alters relative dominance of marine habitats. Marine Ecology Progress Series 289, 5-11. Ryan, J. P., Chavez, F. P., and Bellingham, J. G. (2005). Physical–biological coupling in Monterey Bay, California: topographic influences on phytoplankton ecology. Marine Ecology Progress Series 287, 23-32. Sanchez-Jerez, P., Gillanders, B. M., RodriguezRuiz, S., and Ramos-Espla, A. A. (2002). Effect of an artificial reef in Posidonia meadows on fish assemblage and diet of Diplodus annularis. ICES Journal of Marine Science 59, S59-S68. doi:10.1006/jmsc.2002.1213 Sanchez, F., Serrano, A., and Ballesteros, M. G. (2009). Photogrammetric quantitative study of habitat and benthic communities of deep Cantabrian Sea hard grounds. Continental Shelf Research 29, 1174-1188. doi:10.1016/j.csr.2009.01.004 Sanderson, J. C. (1997). Subtidal Macroalgal Assemblages in Temperate Australian Coastal Waters, Australia. Department of the Environment, Canberra. Schiel, D. R. (2004). The structure and replenishment of rocky shore intertidal communities and biogeographic Temperate reefs literature review 48 comparisons. Journal of Experimental Marine Biology and Ecology 300, 309-342. Schiel, D. R., and Foster, M. S. (1986). The structure of subtidal algal stands in temperate waters. Oceanography and Marine Biology Annual Review 24, 265307. Schiel, D. R., and Taylor, D. I. (1999). Effects of trampling on a rocky intertidal algal assemblage in southern New Zealand. Journal of Experimental Marine Biology and Ecology 235, 213-235. Schmitt, R. J., and Holbrook, S. J. (1990a). Contrasting effects of giant kelp on dynamics of surfperch populations. Oecologia 84, 419-429. Schmitt, R. J., and Holbrook, S. J. (1990b). Population responses of surfperch released from competition. Ecology 71, 1653-1665. Seapy, R. R., and Litter, M. M. (1978). The distribution, abundance, community structure, and primary productivity of macroorganisms from two central California rocky intertidal habitats. Pacific Science 32, 293-314. Sharpe, A. K., and Keough, M. J. (1998). An investigation of the indirect effects of intertidal shellfish collection. Journal of Experimental Marine Biology and Ecology 223, 19-38. Shears, N. T., and Babcock, R. C. (2002). Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia 132, 131-142. Shepherd, S. A., and Clarkson, P. S. (2001). Diet, feeding behaviour, activity and predation of the temperate bluethroated wrasse, Notolabrus tetricus. Marine and Freshwater Research 52, 311322. Sorokin, S. J., Laperousaz, T. C. D., and Collings, G. J. (2008). Investigator group expedition 2006: sponges (Porifera). Transactions of the Royal Society of South Australia 132, 163-172. Sousa, W. P. (1979). Disturbance in marine intertidal boulder fields: The nonequilibrium maintenance of species diversity. Ecology 60, 1225-1239. Spence, S. K., Bryan, G. W., Gibbs, P. E., Masters, D., Morris, L., and Hawkins, S. J. (1990). Effects of TBT Contamination on Nucella Populations. Functional Ecology 4, 425-432. Spencer, R. D. (1970). An ecological study of the subtidal macrophytic vegetation of three selected areas of Port Phillip Bay: Werribee, Altona and Carrum. MSc Thesis, University of Melbourne. Spencer, R. D. (1972). Algal pollution and marine fouling in Port Phillip Bay. PhD Thesis, University of Melbourne. Stephenson, T. A., and Stephenson, A. (1972). 'Life between tidemarks on rocky shores.' (W. H. Freeman: San Francisco.) Stewart, K., Judd, A., and Edmunds, M. (2007). Victorian intertidal reef monitoring program: The intertidal reef biota of Victoria's Marine Protected Areas. Parks Victoria, Melbourne. Suchanek, T. H., Carpenter, R. C., Witman, J. D., and Harvell, C. D. (1983). Sponges as important space competitors in deep Caribbean coral reef communities. In 'The ecology of deep and shallow coral reefs'. (Ed. M. L. Reaka.) pp. 55-60: Washington, D.C.) The University of Queensland (2001). 'Types of reefs.' Available at http://www.reef.edu.au/asp_pages/secc. asp?formno=2. Thomas, F. I. M., and Atkinson, M. J. (1997). Ammonia uptake by coral reefs: effects of water velocity and surface roughness on mass transfer. Limnology and Oceanography 42, 81-88. Thompson, R., Roberts, M., Norton, T., and Hawkins, S. (2000). Feast or famine for intertidal grazing molluscs: a mis-match between seasonal varitations in grazing intensity and the abundance of microbial resources. Hydrobiologia 484, 167-175. Tissot, B. N., Yoklavich, M. M., Love, M. S., York, K., and Amend, M. (2006). Benthic invertebrates that form habitat on deep banks off southern California, with special reference to deep sea coral. Fishery Bulletin 104, 167-181. Underwood, A. (1984). Vertical and seasonal patterns in competition for microalgae between intertidal gastropods. Oecologia 64, 211-222. Underwood, A. J. (1993). Exploitation of species on the rocky coast of New South Wales (Australia) and options for its management. Ocean and Coastal Management 20, 41-62. Underwood, A. J., and Chapman, M. G. (1995). Rocky Shores. In 'Coastal Marine Ecology of temperate Australia'. (Eds A. J. Underwood and M. G. Chapman.). (UNSW Press: Sydney.) Underwood, A. J., and Chapman, M. G. (2007). Intertidal temperate rocky shores. In 'Marine Ecology'. (Eds S. D. Connell and B. M. Gillanders.) pp. 402-427. (Oxford University Press: Oxford.) Underwood, A. J., and Jernakoff, P. (1981). Effects of interactions between algae and grazing gastropods on the structure of a low shore intertidal algal community. Oecologia 48, 221-233. Underwood, A. J., and Kennelly, S. J. (1990). Ecology of marine-algae on rocky shores and subtidal reefs in temperate Australia. Hydrobiologia 192, 3-20. Underwood, A. J., and Keough, M. J. (2001). Supply side ecology: the nature and consequences of variations in the recruitment of intertidal organisms. In 'Marine Community Ecology'. (Eds M. D. Bertness, S. D. Gaines and M. Hay.) pp. 183-200. (Sinauer Associates: Massachusetts, USA.) Underwood, A. J., Kingsford, M. J., and Andrew, N. L. (1991). Patterns in shallow subtidal marine assemblages along the coast of New South Wales. Australian Journal of Ecology 6, 231-249. Vanderklift, M. A., and Wernberg, T. (2008). Detached kelps from distant sources are a food subsidy for sea urchins. Oecologia 157, 327-335. doi:10.1007/s00442-0081061-7 Verges, A., Alcoverro, T., and Ballesteros, E. (2009). Role of fish herbivory in structuring the vertical distribution of canopy algae Cystoseira spp. in the Mediterranean Sea. Marine EcologyProgress Series 375, 1-11. doi:10.3354/meps07778 Wahl, M. e. (2009). 'Marine hard bottom communities: patterns, dynamics, diversity, and change.' (Springer: Heidelberg, Germany.) Waters, J. M., Wernberg, T., Connell, S. D., Thomsen, M. S., Zuccarello, G. C., Kraft, G. T., Sanderson, C., West, J. A., and Gurgel, C. F. D. (In Press). Australia’s Temperate reefs literature review 49 marine biogeography revisited: back to the future? Austral Ecology Waters, J. M. K., T.M.; O'Loughlin, M.; Spencer, H.G. (2005). Phylogeographical disjunction in abundant high dispersal littoral gastropods. Molecular Ecology 14, 2789-2802. Watson, J. E. (1982). Hydroids (Class Hydrozoa). In 'Marine invertebrates of southern Australia. Part I. Handbook of the flora and fauna of South Australia'. (Eds S. A. Shepherd and F. I. M. Thomas.). (Government Printer: South Australia.) Webb, R. O., and Kingsford, M. J. (1992). Protogynous hermaphroditism in the half-banded sea perch, Hypoplectrodes maccullochi (Serranidae). Journal of Fish Biology 40, 951-961. Wheatley, M. J. (2000). Ecology of populations and assemblages of temperate reef fish in Port Phillip Bay, Australia. PhD Thesis, Monash University. Williams, A., Althaus, F., Barker, B., Kloser, R., and Keith, G. (2007). Using data from the Zeehan candidate MPA to provide an inventory of benthic habitats and biodiversity, and evaluate prospective indicators for monitoring and performance assessment. Final Report to the Department of Environment and Water Resources. Williams, A., and Bax, N. J. (2001). Delineating fish–habitat associations for spatially based management: an example from the south-eastern Australian continental shelf. Marine and Freshwater Research 52, 513-536. Williams, A., Bax, N. J., and Kloser, R. J. (2009a). Remarks on "Comment on: Williams et al. (2009) Australia's deep-water reserve network: implications of false Temperate reefs literature review 50 homogeneity for classifying abiotic surrogates of biodiversity, ICES Journal of Marine Science, 66: 214-224" by Peter T. Harris, Andrew D. Heap, Tara J. Anderson, and Brendan Brooke. 66, 2086-2088. Williams, A., Bax, N. J., Kloser, R. J., Althaus, F., Barker, B., and G., K. (2009b). Australia’s deep-water reserve network: implications of false homogeneity for classifying abiotic surrogates of biodiversity. ICES Journal of Marine Science 66, 214-224. Williams, A., and Kloser, R. J. (2004). Voyage plan, RV Southern Surveyor, SS04/2004. CSIRO Marine Research, Hobart. Womersley, H. B. S. (1966). Port Phillip survey 1957-1963. Algae. Memoirs of The National Museum of Victoria Melbourne 27, 133-156. Womersley, H. B. S. (1984). 'The marine benthic flora of southern Australia. Part 1.' (Government Printer: South Australia.) Womersley, H. B. S., and King, R. J. (1990). Ecology of Temperate Rocky Shores. In 'Biology of Marine Plants'. (Eds M. N. Clayton and R. J. King.) pp. 267-295. (Longmire Cheshire: Melbourne.) Wright, J. (1989). An investigation of competition between Cellana tramoserica (Sowerby) and Patiriella exigua (Lamark). Honours Thesis, University of Melbourne. Yoklavich, M. M., Greene, H. G., Cailliet, G. M., Sullivan, D. E., Lea, R. N., and Love, M. S. (2000). Habitat associations of deepwater rockfishes in a submarine canyon: an example of a natural refuge. Fishery Bulletin 98, 625-641. Appendix 1 Tables Table 1. Six bioregions relevant to Victoria as presented in Interim Marine Coastal Regionalisation of Australia Technical Group, IMCRA (2006) Otway Location: Cape Jaffa to slightly north of Apollo Bay and including King Island Very steep to moderate offshore gradients. High wave energy. Currents generally slow, but moderately strong through entrance to Bass Strait. Cold temperate waters subject to nutrient rich upwellings. Central Victoria Location: Cape Otway to west of Wilsons Promontory to Flinders Is. Very steep to steep offshore gradients dominated by cliffed shorelines. Sea-surface temperature is representative of Bass Strait waters. Moderate wave energy. Flinders Location: Eastern Entrance to Bass Strait and including Wilsons Promontory, Rapid changes in offshore gradient. Granitic coastline exposed to submaximal swells on east-facing shores of Flinders Island and moderate to low swells elsewhere. Sandy beaches of moderate length with seagrass beds prevalent in shallow water. High tidal range » 3 m and strong tidal currents. Sea-surface temperature is representative of Bass Strait waters. Waves highly variable. Twofold Shelf Location: East of Wilsons Promontory and north to Tathra (36°48’S), Submaximally exposed coastline with long sandy beaches broken by rocky headlands and numerous coastal lagoons. Moderate tidal range ~ 2 m. Mean annual sea-surface temperature reflects the influence of warmer waters brought into Bass Strait by the East Australian Current. Variable wave energy. Victorian Embayments Location: Victorian bays, inlets and estuaries e.g., Port Phillip Bay Confined bodies of water that total in excess of 3000 km 2 and individually vary in size from 1950 km2 to less than 1 km2. They are generally basin-shaped, less than 25 m deep, have limited fetch and are dominated by depositional environments. Central Bass Strait Location: Central offshore region of Bass Strait The region is about 60,000 km2 in size and lies in the central area of Bass Strait. The sea floor is shaped like an irregular saucer with water depth varying from about 80 m at its centre to 50 m around the margins. The substrate of central area is mainly mud. Tidal velocities vary from <0.05 ms–1 in the central area to as high as 0.5 ms–1 at the margins where the islands and promontories form the western and eastern entrances to Bass Strait. Water mass characteristics are complex and vary seasonally representing the mixing of the different water masses present on western and eastern side of the Strait. Temperate reefs literature review 51 Table 2. Interim intertidal marine habitat (MHC) categories for Victoria, Ferns et al. (2000). Table 3. Interim shallow subtidal (0 – 2.5 m) marine habitat (MHC) categories for Victoria, Ferns et al. (2000). Temperate reefs literature review 52 Table 4. Primary shallow habitat classification scheme as presented in Ball et al. (2000). Temperate reefs literature review 53 Table 5. Potential physical drivers responsible for shaping deep reef assemblages in Victoria. Summarised from Edmunds et al. (2006b) o o o o o Sedimentation Sediments may naturally settle and cover rock Strong wave action and currents may expose rocky reefs and keep them free of sediments With increasing depth, wave action decreases and sedimentation may increase Sediment transport from adjacent habitats may be such that reefs are heavily sediment effected even in the presence of strong currents Reef steepness and relief can influence sediment build-up, i.e., more vertical surfaces are less likely to accumulate sediment Geological structure The type of rock and geological history can influence the size and structure of reefs Surface roughness can effect settlement, increase surface area and influence water movement over the rock surface and therefore the transport of food, oxygen, nutrients etc (Thomas and Atkinson 1997) Reef structure may influence how sediments and loose rocks smother or scour reef surface and sessile organisms Water movement, including waves and currents May erode reefs Can impact sediment deposition and transport Influence the type and distribution of organisms on the reef Light climate High levels of sedimentation and increasing depth can influence light levels Reef aspect and shading at a variety of scales influence light availability Where light levels are sufficient, algae out compete sessile invertebrates on rocky reefs, e.g., sponges and corals Depth Interacts with factors listed above Deep reefs tend to be dominated by sessile invertebrate assemblages, e.g., sponge gardens Temperate reefs literature review 54 Table 6. Potential ecological drivers responsible for shaping deep reef assemblages in Victoria. Summarised from Edmunds et al. (2006b) Sessile invertebrates spend their adult life attached to the substratum o Species such as sponges, may exhibit different physical characteristics depending on environmental conditions o Sponges require suitable seabed for them to attach and resist the actions of currents and waves and are often found in underwater tunnels and notches that are surrounded by kelps (Keough 1999) o Sponges, cnidarians, bryozoans, and colonial ascidians dominate deep reef assemblages o The age of individuals and colonies on deep reefs in Victoria cannot be assumed to be correlated with size o There may be complex interactions which will not be known without study of the basic ecology of species, o Sessile clonal species such as sponges often release actively swimming larvae which travel relatively short distances before recruitment, even in high currents , while some species produce crawling larvae o Larvae can react to a range of stimuli such as light levels and gravity (Keough et al. 1996) Food availability effects the distribution of species, and while mobile invertebrates and fish can move to areas where food is available, sessile species are predominantly suspension feeders that require food in the form of plankton that is available in the water column. Little is known about the small crustaceans, molluscs etc that are associated with sessile communities on Victoria’s deep rocky reefs. o Therefore their role in shaping assemblage structure is not understood. Little is known about the behaviour and diet of predatory fish on Victoria’s deep reefs, or their impact on assemblage structure Temperate reefs literature review 55 Appendix 2 Conceptual Models Temperate reefs literature review 56 Temperate reefs literature review 57 Figure 1. Key relationships and drivers on intertidal reefs in Victoria Temperate reefs literature review 58 Figure 2. Key relationships and drivers on subtidal reefs in Victoria Temperate reefs literature review 59 Figure 3. Key relationships between invertebrates on subtidal reefs in eastern and western Victoria Temperate reefs literature review 60 Figure 4. Key relationships and drivers on deep reefs in Victoria Temperate reefs literature review 61 Figure 5. Key relationships and drivers on canyon reefs in Victoria Temperate reefs literature review 63