UV spectrum of 16 in acetonitrile

# Supplementary Material (ESI) for Chemical Communications
# This journal is © The Royal Society of Chemistry 2004
Supplementary Information
A highly congested N,N’-dioxide fluorosensor for enantioselective recognition of chiral hydrogen bond donors
Xuefeng Mei and Christian Wolf*
Department of Chemistry, Georgetown University, Washington, DC 20057, USA.
General Procedures
All reactions were carried out under nitrogen. Commercially available reagents and solvents were used without further purification. Flash chromatography was performed on silica gel (particle size 0.032-0.063mm). NMR spectra were obtained at 300 MHz ( 1 H
NMR) and 75 MHz (
13
C NMR) using CDCl
3
as the solvent. Chemical shifts are reported in ppm relative to TMS. Elemental analysis data were collected using a Perkin Elmer
2400 CHN. Diacridylnaphthalene 9 was prepared according to a literature procedure.
1
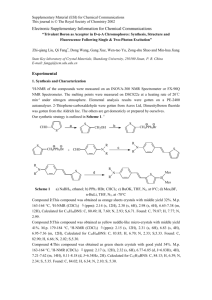
Preparation of anti-1,8-bis(3,3’-(3,5-dimethylphenyl)-9,9’-diacridyl)naphthalene
N,N’-dioxide, 1
. A solution of anti 9 , (100 mg, 0.15 mmol) in 3 mL of THF was treated with perbenzoic acid (68 mg, 77% purity, 0.30 mmol) in 2 mL of THF at room temperature. The mixture was allowed to stir at room temperature for 5 hours and the solvent was removed by evaporation under reduced pressure. The residue was dissolved in methylene chloride and washed with 2N sodium hydroxide, dried over MgSO
4
and concentrated in vacuo. Purification by flash chromatography (100:10 ethyl acetate:ethyl alcohol) afforded 1 (80 mg, 75 %) as a red solid.
1
H-NMR (300 MHz, CDCl
3
)
= 2.45
(s, 12H), 6.63-6.69 (m, 2H), 6.81 (d, J = 9.1 Hz, 2H), 6.87 (d, J = 8.0 Hz, 2H), 7.09-7.14
(m, 4H), 7.34-7.41 (m, 8H), 7.77 (dd, J = 7.2 Hz, 8.2 Hz, 2H), 8.32 (dd, J = 1.1 Hz, 8.2
Hz 2H), 8.47 (d, J = 9.1 Hz 2H). 8.69 (d, J = 1.7 Hz, 2H).
13
C-NMR (75 MHz, CDCl
3
)
= 22.3, 117.6, 120.5, 126.1, 126.5, 126.5, 126.6, 126.7, 126.3, 126.9, 127.5, 130.0, 130.7,
131.0, 132.2, 133.4, 134.1, 135.1, 135.9, 138.6, 138.6, 139.2, 140.2, 143.0. Anal. calcd. for C
52
H
38
N
2
O
2
: C, 86.43; H, 5.26; N, 3.88. Found: C, 86.40; H, 5.33; N, 3.76.
1
HPLC Enantioseparation of 1.
Chiral HPLC was carried out on an HP 1050 equipped with an autosampler and DAD detector to separate the enantiomers of 1 on a Chiralpak AD column (250 mm x 4.6 mm,
5
m) using hexanes/EtOH (2:3) as the mobile phase. The levorotatory enantiomer was eluted first. The HPLC enantioselectivity factor,
, was determined as 1.70. Preparative separations were performed by repetitive injections of 50
L of 1 dissolved in hexanes/EtOH (1:1) at a concentration of approximately 10 mg/mL. For analytical separations, 10
L of solutions containing 1 dissolved in the same diluent at a concentration of 1 mg/mL were injected. Semi-preparative HPLC enantioseparation on
Chiralpak AD allowed determination of the specific rotation by polarimetry as [
] 25
589
= -
520 (1 st eluted enantiomer, c = 46 mg/100 mL CH
2
Cl
2
) and +515 (2nd eluted enantiomer, c = 39 mg/100 mL CH
2
Cl
2
). Specific rotations were determined on a Rudolph
Instruments Digipol 781 polarimeter. Circular dichroism spectra of the enantiomers of 1 were obtained at 5x10
-6
M in acetonitrile on a JASCO J-710 circular dichroism chiroptical spectrometer.
(-)1
= 1.70
(+)1
-1011563114666217769311086
0 2 4 6 8 10 12 14 min
Figure 1. Enantioseparation of 1 on Chiralpak AD. Diluent: hexanes/EtOH = 1:1; mobile phase: hexanes/EtOH = 2:3; UV detection: 254 nm; injection volume: 10
l; sample concentration: 1 mg/mL.
Fluorescence spectrum of 1
Fluorescence experiments were conducted using a Fluoromax-2 from Instruments S.A.
Inc. Absorption and emission spectra were collected under nitrogen using a 3.5 10
-5
M solution of (+)1 in acetonitrile. Excitation wavelength was 490 nm and emission wavelength was 571 nm.
2
16000
12000
AU 8000
4000
0
490 540 590 640 690
/nm
Figure 2. Fluorescence spectrum of anti-1 in acetonitrile.
Fluorescence titration of (-)-1 in the presence of the enantiomers of 10 in acetonitrile
2.2
2
1.8
I
0
/I
1.6
1.4
1.2
y = 0.0914x + 1
R 2 = 0.9883
( R )10
( S )10 y = 0.0672x + 1
R 2 = 0.9897
1
0 5 10 15 c/mmol
The concentration of (-)1 was 3.5x10
-5
M. Excitation wavelength: 490nm; emission maximum: 571nm.
Fluorescence titration of (+)-1 in the presence of the enantiomers of 11 in toluene
1.1
1
0.9
0.8
I
0
/I
0.7
0.6
0.5
( S, S)11 y = -0.0324x + 1
R 2 = 0.984
( R,R )11 y = -0.052x + 1
R 2 = 0.9988
0.4
0 2 4 6 8 10 c/mmol
The concentration of (+)1 was 7.5x10
-6 M. Excitation wavelength: 490nm; emission maximum: 571nm.
3
Benesi-Hildebrand equation
I
0
= b 1
+ 1
I-I
0 a-b K [M] where I
0
is the inherent fluorescence intensity of (+)1 , I is the fluorescence intensity in presence of an analyte, [M] is the analyte concentration, and K is the association constant, a and b are constants.
Benesi-Hildebrand plots
35
0
-2
30
-4
-6
(+)1 + ( R )10 25
(+)1 + ( S,S )11
I
0
I-I
0
-8
-10
-12
(+)1 + ( S )10
I
0
I-I
0
20
15
10
5
(+)1 + ( R,R )11
-14
-16 0
0 200 400 600 0 200 400 600
1/[M] 1/[M]
Figure 3. Benesi-Hildebrand plot of (+)1 in presence of the enantiomers of 10 (left) and
11 (right). The concentration of (+)1 was 7.5x10
-6
M. Excitation (emission) wavelength:
490 nm (571 nm).
1 C. Wolf, X. Mei, J. Am. Chem. Soc.
2003, 125 , 10651-10658.
4