FABRICATING AND CHARACTERIZATION OF Cu2O/TiO2
advertisement

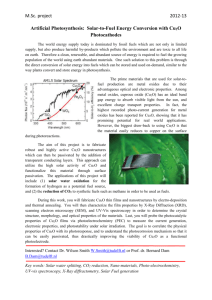
World Journal of Engineering FABRICATION AND CHARACTERIZATION OF Cu2O/TiO2 COMPOSITE FILMS FOR SOLAR CELL APPLICATIONS Ayib Rosdi Zainun1,2,3, Tomoya Sakamoto1, Uzer Mohd Noor2, Mohamad Rusop2, Masaya Ichimura1 1 Department of Engineering Physics, Electronics and Mechanics, Nagoya Institute of Technology, Nagoya 466-8555, Japan. Solar Cell Laboratory, Faculty of Electrical Engineering, Universiti Teknologi MARA (UiTM), Shah Alam, Selangor, Malaysia. 3 Faculty of Electrical &Electronics Engineering, Universiti Malaysia Pahang (UMP), Kuantan, Pahang, Malaysia. 2 needs to flow through the TiO2 matrix. However, the TiO2 particles composing the matrix are resistive, and therefore the current would preferentially flow from the film/substrate interface into the deposition solution. Thus Cu2O was preferentially deposited near the interface rather than on the top surface. Fig. 3 shows the X-ray diffraction spectra of the Cu2O, TiO2 and Cu2O/TiO2 composite films. All the peaks observed for TiO2 are attributed to the TiO2 anatase structure, and those observed for Cu2O are attributed to the Cu2O cubic structure. For the composite film, in addition to the TiO2 peaks, the Cu2O peaks are observed. Introduction Use of oxide semiconductors such as Cu2O and TiO2 as alternatives to silicon for solar cells attract much attention among the researchers. Cu2O is a p-type semiconductor with a direct band gap of 2.1 eV and is regarded as a suitable material for high-efficiency solar cells [1-4]. TiO2 is an n-type semiconductor with a wide band gap energy of 3.2 eV and known for its photo catalytic effects [5], and it has been widely used for anti-fouling coating and dye-sensitized solar cells [6]. The combination of these two materials could contribute to efficient photoelectric conversion. Siripala et al. fabricated a Cu2O/TiO2 heterojunction thin film and observed its photoresponse in a photoelectrochemical cell [7]. In this research, Cu2O/TiO2 composite thin films have been fabricated by combination of squeegee and electrochemical deposition (ECD) methods, and a cell has been fabricated by forming metal electrodes on the film. Structural and optical properties of the films have been characterized, and photoresponse of the cell has been measured. Experimental Procedures (a) TiO2 films with a thickness around 16 μm were prepared by the squeegee method using 0.8 g/mL TiO2 paste of TiO2 powders (P25, Aerosol Japan) with addition of 0.5 mL acetyl acetone. The paste was mixed and blended with 0.4 g polyethylene glycol and 2.5 mL triton X for about 5 min in each process. Then the TiO2 films were heated and annealed at 100oC and 400oC for 30 min in air. The substrate is Fdoped SnO2 (FTO) coated glass. The deposition of Cu2O on the TiO2/FTO substrate by ECD was conducted using an aqueous solution containing 0.5 mol/L copper (II) sulfate and 6 mL lactic acid in 20mL of pure water. The solution pH was adjusted to 12.5 with KOH. The galvanostatic electrochemical deposition on the TiO2/FTO substrate was carried out at a current density of about -1 mA/cm2, and the deposition time is 10 min unless otherwise stated. (b) (c) Fig. 1 Appearance of (a) the TiO2 film, and (b) the front surface and (c) the back surface of the Cu2O/TiO2 composite film. TiO2 Cu2O FTO Glass Fig. 2 Hypothesis of the Cu2O/TiO2 film structure. Cu2O/TiO2 Intensity (a.u.) Result and discussion Physical appearances of the films are shown in Fig. 1. After the Cu2O deposition, the front surface remained white, while the back side (substrate side) became orange (color of Cu2O). Thus Cu2O seems to penetrate the TiO2 film and be dominantly deposited near the TiO2/FTO interface rather than on the TiO2 film surface. This would be because Cu2O gradually filled the porous matrix of TiO2. from the bottom as in Fig. 2. During the deposition of Cu2O, the deposition solution would easily penetrate the TiO2 film. For Cu2O to deposit on the top surface of the TiO2 film, the electric current (101) (004) TiO2 (110) (111) Cu2O 10 20 30 40 50 60 2 (deg.) Fig. 3 X-ray diffraction spectra of the Cu2O, TiO2 and Cu2O/TiO2 composite films. 1295 World Journal of Engineering 10 min-deposition sample, a typical solar cell characteristics were observed, and the short-circuit current is 0.0031 mA/cm2, the open-circuit voltage 0.47 V, and the efficiency 5x10 −4 %. Thus a rectifying pn junction was formed with the front surface side acting as n-type and the film/substrate interface side acting as p-type. This will be because the surface side is dominantly TiO2 and the interface side dominantly Cu2O, as shown in Fig.2. A solar cell based on a mixture of n-type and p-type semiconductors are commonly called a blend solar cell or a bulk-heterojunction solar cell. In all previous works on bulkheterojunction solar cells, the photovoltaic blend film consisted of two organic semiconductors, or one organic and one inorganic semiconductors. We demonstrated that the Cu2O/TiO2 composite film showed photovoltaic behavior, and thus we can regard our composite film as an inorganicinorganic bulk-heterojunction thin film. 100 Transmission (%) 80 60 Cu2O 40 TiO2 20 Cu2O/TiO2 0 800 700 600 500 Wavelength (nm) 400 300 Fig.4 Optical transmission spectra for the TiO2, Cu2O, and Cu2O/TiO2 composite films. Fig. 4 shows optical transmission spectra for the TiO 2, Cu2O, and Cu2O/TiO2 composite films. The Cu2O film has an absorption edge around 570 nm, corresponding to its band gap of 2.1 eV. The TiO2 film is porous and thus the transmission is low in the visible range because of scattering. The absorption edge is observed near 400 nm. For the composite films, the transmission is null for wavelengths shorter than 520 nm because of the absorption by Cu2O. Conclusions Cu2O films have been deposited by ECD on TiO2 films prepared by the squeegee method, and a cell has been fabricated by evaporating In on the film. By I-V characterization, the cell showed electrical rectification and photovoltaic effects. Even though the overall performance of the cell has not been well optimized yet, we have demonstrated that an inorganic bulk heterojunction solar cell can be fabricated by a simple approach based on the ECD and squeegee methods. (−) Indium (+) Acknowledgements Cu2O/TiO2 composite film Special thanks to all members of Prof. Ichimura lab. for their useful discussion, cooperation, support and assistance in completing this paper. FTO Glass Fig. 5 Position of the electrodes for the I-V measurement. References 1.E-03 5 min 1. Izaki, M., Shinagawa, T., Mizuno, K. T., Ida, Y., Inaba, M. and Tasaka, A. Electrochemically constructed p-Cu2O/nZnO heterojunction diode for photovoltaic effect. J. Phys. D: Appl. Phys., 40 (2007) 3326-3329. 2. Mittiga, A., Salza, E., Sarto, F., Tucci, M. and Vasanthi, R. Heterojunction solar cell with 2% efficiency based on a Cu2O substrate. Appl. Phys. Lett., 88 (2006) 163502. 3. Cui, J. and Gibson, U. J. A Simple Two-Step Electrodeposition of Cu2O/ZnO nanopillar solar cells. J. Phys. Chem. C, 114 (2010) 6408-6412. 4. Zhou, B., Liu, Z., Wang, H., Yang, Y., and Su, W. Experimental study on photocatalytic activity of Cu2O/Cu nanocomposites under visible light. Catal. Lett., 132 (2009) 75–80. 5. Fujishima, A. and Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature, 238 (1972) 3738. 6. Gratzel, M. Dye-sensitized solar cells. J. Photochem. Photobio. C, 4 (2003) 145-153. 7. Siripala, W., Ivanovskaya, A., Jaramillo, T. F., Baeck, S. H. and McFarland, E. W. A Cu2O/TiO2 heterojunction thin film cathode for photoelectrocatalysis. Sol. Energy Mater. Sol. Cells, 77 (2003) 229-237. 15 min 0 2 Current (mA/cm ) 0.E+00 0.1 0.2 0.3 0.4 0.5 0.6 0.7 -1.E-03 -2.E-03 10 min -3.E-03 -4.E-03 Voltage (V) Fig. 6 I-V characterization of the Cu2O/TiO2 composite films under AM1.5 illumination of 100mW/cm2. For conducting the I-V characterization, indium was evaporated as shown in Fig. 5. Fig. 6 shows the photovoltaic effects of the Cu2O/TiO2 composite films measured during illumination through the FTO glass substrate. Three types of samples with different Cu2O deposition times were prepared and measured for comparison. The AM1.5 light intensity was maintained at 100mW/cm2 for all the measurements. For the 1296