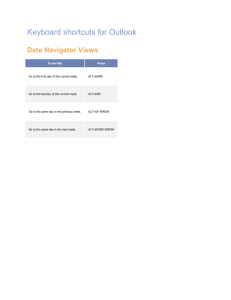
Diagnostic Enzymology
advertisement

Diagnostic Enzymology Fourth Year Medicine Enzymes can be used as “markers” to detect and localize cell damage or proliferation [(1) enzymes are present at much higher concentrations inside cells than in plasma, (2) some enzyme occur predominantly in certain types of cell] In “normal” healthy individuals (where there is a steady cell turnover), enzyme activity in plasma/serum represents: a balance between its rate of liberation into the Extracellular (E.C.) space and its rate of clearance/uptake from E.C. space (+ auto degradation of enzyme) General cases in which [Enzymes] in plasma are elevated: a. Cell proliferation: There is an increase in cell turnover. b. Non-proliferative increase in the rate of cell turnover. c. Cell damage. d. Enzyme induction. Mechanisms of Elevated Enzyme Activities: Cell membrane crucial function in retaining cytosolic enzyme within cells. 1. ATP role (maintenance of cell permeability): In the absence of added ATP to medium containing cells, the rate of escape of intracellular enzyme is strictly related to the rate of depletion of intracellular ATP. Anoxia (e.g. in severe cardiac / respiratory disease) may allow escape of intracellular enzyme from cells even in the absence of organ damage. Hypoxia (e.g. during severe exercise) may allow transient escape of enzymes from muscle cells. 2. Drugs / Poison: Inhibitors of energy-yielding processes (e.g. chlorpromazine and promethazine), exert direct effect on cell membrane to accelerate enzyme release. Bacterial toxins (e.g. generalized septicemia) cause increases in serum enzyme activities. ** The role of electrolytes and osmotically active molecules in maintaining cell integrity is evident. ∴ One can see why in some cases serum enzymes elevations are non-specific. ?! Hence: Look for indices directly related to actual cell necrosis: * Mitochondrial enzymes: Only liberated during cell necrosis e.g. Glu-DH, AST (exist in mitochondrial cytosolic form). form, distinct from 3. Lipid perioxidation: affect release of membrane – associated enzymes 4. Hepatic (microsomal) enzyme induction: ALP, GGT, 5-nucleotidase are elevated in epileptic patients taking anti-convulsants. Also, in biliary obstruction: bile acids promote liberation of enzymes from damaged hepatic cell 5. The enzyme rate of clearance and elimination Half-lives of: ALT- 6.3 days AST- 2.0 days CK- 1.4 days The rate of enzyme clearance depends on its rate of elimination from serum. Different mechanisms apply: Amylase and lipase Urine (detection of urinary amylase is essential in acute pancreatitis) ALP excreted partly in the bile. Reticuloendothelial system clears some enzymes. Proteolytic degradation. Non-biologic decay (same as where serum specimens are not kept refrigerated). Conjugation of enzyme with Abs (enzyme loses its biological potency) e.g. CK/LDH Measurement of Enzyme Activities Enzyme units: IU: µmol of substrate transformed per minute under standard conditions. New unit: Katal: amount of activity that convert one mole of substrate/second (1 Katal = 6x107 IU) Some Important Enzymes Clinically: Acid phosphatase (ACP): hydrolysis of a wide range of phosphate ester bonds at acid pH. The activity of ACP decreases rapidly at room temperature unless pH is reduced below 6.0. Isoenzyems of ACP: a series of ACPs exists in all body cells. Soluble form in the cytosol, but the lyosomal is the predominant form. Important tissue sources: RBCs, platelets, Prostate. Prostate enzyme is sensitive to L-tartrate RBC enzyme is sensitive to formaldehyde (* in hemolyzed blood sample in the presence of formaldehyde ACP release is inhibited). Other tissue forms of ACP are sensitive to L-tartrate (detectable in females and in males after prostatectomy). Clinical Applications: The main use of ACP lies in the diagnosis of prostatic cancer (only when the disease has spread to adjacent bone e.g. pelvis). The enzyme in serum is markedly increased. Spread to soft tissue: high ACP level may be seen. If tumor remains as nodule / not extended beyond prostate capsule: only ½ of patients may show elevated serum activity of ACP. Therefore, ACP use as diagnostic and screening test is limited in prostatic cancer. The use of “prostate specific antigen” (PSA) is a recent method for P.C. diagnosis and screening. Prostatic massage: rectal examination may cause (rarely) transient elevation of ACP. Because of enzyme location predominantly in lysosome, raised serum ACP activity is expected in some liver storage diseases (e.g. Gaucher’s disease, Niemann-Pick disease). Acute retention of urine and passage of catheter can cause raised serum ACP activity. ACP may be elevated in thromboembolic and myeloproliferative disorders. Alkaline Phosphatase (ALP): Low substrate specificity catalyzes hydrolysis of phosphate esters at alkaline pH. All ALPs contain Zn and Ser at active site. Cellular Location: 1) Brush borders of proximal convoluted tubule of kidney and intestinal mucosa. 2) Other membranes include: Sinusoidal and Canalicular surfaces of hepatocytes. Its location in membranes plays a role in absorption and transport processes. 3) Bones: ALP role in minerlization since its activity changes with osteoblastic function. Isoenzymes: Placental and small intestinal mucosa: ALP forms under separate genetic control. Bone, liver and kidney: ALP is a product of the same gene locus but subject to post-translational modification within each tissue. Θ Liver • Bone • Placenta • Intestine • Effect of Heat: Placental ALP is unaffected at 65˚ C, whereas all other ALP isozymes lose their activity totally. Heating at 56˚ C for 10 minutes: Intestinal ALP loses 20% of its activity, liver ALP 60% and bone ALP 80%. Urea denaturation (2M): Placental ALP most stable, bone ALP the least. Physiological causes for raised serum ALP: 1. In infancy due to predominance of bone ALP until about the age of puberty (2-2 ½ x adult normal). In adult most ALP activity is due to liver isoenzyme. 2. Intestinal ALP appears only in serum if a person is of blood groups O or A, secretors of ABH red cell antigens and are positive for Lewis antigen. 3. At puberty: up to 5-6 x adult age. 4. Pregnancy: placental ALP appears in serum of pregnant women only during second and third trimester. Sharp reduction in P.ALP often indicates placental insufficiency and death of fetus. Clinical Applications: 1. Hepatobiliary Disease A. Very high levels of ALP are seen in biliary obstruction Moderate levels of ALP are seen in parenchymal liver disease This is important in differential diagnosis of hepatic and obstructive jaundice. B. In extrahepatic obstruction: serum bilirubin and ALP rise and fall in parallel. C. In acute hepatic necrosis there is little change in ALP even when bilirubin concentration is rising dramatically. If icterus fails to clear and patients go into cholestatic phase, ALP rises substantially. D. In biliary cirrhosis: high level of ALP even when bilirubin is normal. However, in portal cirrhosis ALP level remains normal. E. In alcoholic fatty liver: ALP is raised. F. In carcinoma or lymphatous infilteraton of liver, the Regan isoenzyme (resembles placental ALP) has been detected in 1% - 3% of such patients (especially in bronchial carcinoma). There are various tumor–associated ALP but their importance is in providing insight into genetic changes occurring during neoplasia, rather than as aids to diagnosis. 2. Bone Disease A. Primary bone tumors (e.g. osteogenic sarcoma) and secondary osteoblastic bone tumors (e.g. those originating from prostate): can cause very high ALP level. However, osteolytic secondaries show slight to moderate increase of ALP. B. Paget’s disease: high ALP. C. Osteomalacia and rickets (vitamin. D deficiency): high ALP. D. Primary hyperparathyroidism with bone involvement: often associated with high ALP. E. Secondary hyperparathyoidism, as in renal ostesodystrophy: Dramatic increase in ALP. F. Healing fractures: high ALP. 3. Intestinal disease A. High level of ALP (bone) found after gastrectomy due to impaired intestinal absorption of Ca++, PO4--, and vitamin D. Creatine Kinase (CK): Creatine + ATP Creatine – P + ADP Tissues: mainly brain, skeletal and smooth muscles. Physiological increase: Males > Females, increase with age and body weight Neonates but fall rapidly during the first weeks. Physical exercise. Labour and parturition. Isoenzymes: 1. BB- Brain: moves rapidly towards 2. MM- Skeletal / Heart muscles: slow moving 3. MB- Comprises 20-40% of CK found in heart also found in small amounts in red fibers. Other isoenzymes e.g. x.y.z. Clinical Applications: 1. Myocardial Infarction (M.I.): The most useful test in establishing / refuting M.I. Increase in serum CK after 6 hours of M.I. onset reaching a peak after 36 hours, declining to normal value after day 3. Note: Re-infarction, thromboembolism, and arrhythmias may cause a delay in return to normal value or cause secondary spikes. The following conditions can also cause moderate increase in serum level of CK: A. Prolonged chest pain with transient ST-segment and T-waves changes (40% of cases). B. Prolonged chest pain with E.C.G. abnormalities (20% of cases). C. Classic angina pectoris (Occasionally). The significance of MB isoenzyme in M.I. (prognostic Value): There is about 2% of CK present in normal serum is of MB-type. After infarct within few hours MB increases dramatically but with shorter duration (due to its rapid clearance from vascular space). The increase in the amount of CK-MB determines the severity of M.I. and hence prognosis. 2. Muscle Disease: A. Duchenne Muscular Dystrophy: CK is elevated mainly CK-MM. Other enzymes are elevated: Aldolase, LDH, aminotransferases. B. Becker Muscular Dystrophy: more benign. forms of muscular dystrophy: lesser elevation. C. Polymyositis. Other D. Secondary diseases of muscles: e.g. neurogenic atrophy and malignant hyperpyrexia. 3. Cerebral Disease: A. Epileptic seizure due to muscular activity. B. Tetanus C. Organic neurologic disease e.g. cerebral infarction, meningitis and cephalitis (Ck - BB). 4. Other conditions: elevated in alcohol intoxication, hypothyroidism. Lactate Dehydrogenase (LDH) (NAD- Oxidoreductase): Zn-containing enzyme, catalyzing the following reversible reaction: pyruvate + NADH+H+⇌Lactate + NAD+ Tissue Distribution: throughout all body tissues (in cytosol) with high concentrations in: heart, skeletal muscle, liver, kidney, brain, RBCs. Therefore, it is a non-specific index of cell damage. Since its presence in RBCs, hemolysis or delayed separation of plasma cause artefactual increase activity of LDH. Isoenzymes: 5: LD1 LD5 LD1 (H4), LD2 (H3M), LD3 (H2M2), LD4 (HM), LD5 (M4). LD1: moves fastest to, while LD5 is the slowest. Clinical Applications: 1. Myocardial Infarction (M.I.): (>5 x normal). Elevation commences 12-18 hours after onset of symptoms, peak on the third day; the activity then declines until reaching normality by the 10th day (in uncomplicated cases). LDH assay is more valuble in a case presenting several days after suspected M.I. Heart Muscle is rich in LD1 and LD2 (LD1>LD2): In normal serum LD2> LD1 In M.I. “flipped” pattern of LD occurs: LD1>LD2 in serum. 2. Hepatobiliary Disease: Increased serum LDH in acute liver necrosis. LDH levels are raised in most forms of hepatobiliary disease including obstructive jaundice, and their discriminatory capacity is poor. LD5 is the predominant isoenzyme. 3. Blood Disease: Marked elevation (>5x) in megaloblastic anemia, and leukemias. Main increase in LD1 and LD2 (red nucleated cells in bone marrow is the source of the increase). In hemolytic anemia LD1 and LD2 are liberated directly from circulating RBCs. 4. Cancer: High serum LDH is associated with widespread metastases especially to liver. LD4, LD5 are the predominant isoenzymes. The Aminotransferases (ALT/AST): Ala + α KG ALT Pyruvate + Glu Asp + α KG AST oxloacetate + Asp Tissue Distribution: ALT: Almost exclusively found in the cytosol and in high concentration in hepatocyte. AST: found in cytosol and mitochondria. Abundant in: liver, heart, and skeletal muscle. Clinical Applications: 1. Myocardial Infarction: AST (markedly elevated >10x) is usually detectable 6-12 hours after onset of M.I., reaching a peak 20-48 hours after onset of symptoms, falls thereafter, returning to reference value by the 5th day. Delayed fall in ASP activity may be due to: heart failure. In very severe M.I. mitochondrial AST may be detected in serum (prognostic index). ALT (moderately elevated) in uncomplicated M.I. In event of cardiac failure, severe shock or other complications, ALT may rise >10x normal value. This is attributed to release of ALT from damaged hepatocytes due to tissue anoxia. ALT is of little diagnostic value in M.I. but useful in detecting the complication that develop later. 2. Hepatobiliary Disease: In viral hepatitis (V.H.): Both ALT and AST are markedly increased (10-100 x normal) Elevation starts from prodormal phase up to 10 days before icterus (jaundice) is evident. ALT and AST are useful in studying the epidemiology of V.H. In early genesis of V.H.: Serum AST>ALT since [AST] in liver is higher than [ALT]. Later, serum ALT>AST due to slower clearance of ALT (λ ½ = 6.3 days) Continuing elevation of serum ALT and AST is indication of the presence of: Chronic active hepatitis. If elevation is moderate, serum AST: ALT < 1: Chronic persistent hepatitis is more likely. If elevation is higher than moderate and AST: ALT > 2: Chronic aggressive hepatitis is likely and prognosis is less favorable. In Portal cirrhosis: Both ALT/AST is normal if icterus is absent, and slightly raised if present. Elevation rarely more than 3x normal (AST>ALT). In biliary cirrhosis: morderately pronounced (up to 4 x n) increase (ALT=AST) In hepatobiliary obstruction: range of AST/ALT is 2-8 x normal (depending on degree of icterus). When obstruction is caused by non-malignant disease, ALT>AST (usually). In a proportion of cases, where jaundice is caused by tumor AST>ALT. In hepatic metastases half of patients show AST is the dominant aminotransfrase. Major disadvantage of Aminotransferases: Lack of Specificity. Other enzymes of diagnostic importance: 5 NT, GGT, plasma and urinary -amylase, lipase, trypsinogen and trypsin.