Eighth_Physical_Science_Unit - Forest Ridge Elementary School
advertisement

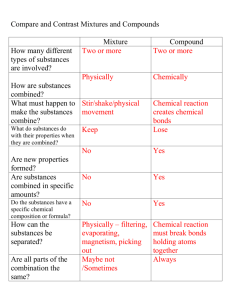
SCIENCE: Forest Ridge School District 142 Properties of Matter: Eighth Grade Unifying Theme: Evidence & Explanation Essential Question: How can we support our explanations (claims) with supporting evidence gathered through scientific investigation and technological design? Guiding Questions: How can we identify substances based upon their characteristic properties? How can we distinguish between elements, compounds, mixtures and solutions? How can we distinguish between physical and chemical changes? How can we use the concept of atoms to explain conservation of matter? How can we use the properties of matter to create a system of organization? How has our study of nanoscale impacted technological advances? Big Ideas Substances have characteristic properties all of which are independent of the size or phase of the sample. A mixture of substances can be broken down into the original components, or elements, showing one or more of its characteristic properties. (NSES) There are more than 100 known elements. They are able to combine in many ways to produce compounds that make up the living and nonliving substances on Earth. (NSES) Technological advances Power Standards Analyze the characteristic properties of matter, recognizing that a mixture of substances can be broken down into original components showing one or more of its characteristic properties. Descriptors: Based on National & State Documents Classify objects based on their physical and chemical properties. Determine an unknown by analyzing its properties. (i.e. mixtures, solutions, pure substances, acids, bases, reactivity, phases) ASSESSMENT Formulate an explanation of phase changed based on the transfer of energy. Compare positions, movements and relationships of molecules in different phases as an interaction with thermal energy transfer. Generate an explanation that provides evidence that all living and nonliving things are composed of different combinations of elements. Analyze and classify substances as pure substances or mixtures based upon their properties. Formative Assessmen Support a claim w Diagram - The re molecules, eleme Quiz – Formulate relationships amo and compounds. Synthesis of perio Peer Practice - Ar element on the p chemical characte Chemistry Quiz – Conservation of M Formulate an explanation of the relationships among atoms, molecules, elements and compounds. Analyze conservation of mass and energy. Argue the placement of an element on the periodic table based upon its chemical characteristics. Describe density as a relationship between mass and volume. Describe how nanoscale has impacted technological advances. Summative Assessme Argue the placement of based upon its chemical Summative Assessme Develop an i-movie, stor communicating how nan engineering and/or Cost Nanotechnology. in matter are being made on the nanoscale. Scientific Inquiry Scientific inquiry is a dynamic process that is not limited to one scientific method. Formative Assessmen Define Nanoscale Synthesize resea T Chart Cost/Ben Propose scientific questions and engage in active inquiry gathering and prioritizing evidence, formulating explanations, making connections to scientific knowledge and communicating and justifying explanations. Design and conduct a scientific procedure to determine the identity an unknown substance. Report, display, and defend, to an audience conclusions drawn from an investigation. Inquiry engages learners in asking scientifically oriented questions, gathering and prioritizing evidence, formulating explanations, making connections to scientific knowledge and communicating and justifying explanations. Engineering Design systems have interacting parts that can be analyzed by investigating the complexity of inputs and outputs and their relationship to the feedback loop. Technological design/engineering is a dynamic problem-solving process that engages designers in proposing solutions, testing, analyzing and modifying. The Nature of Science Science is an imaginative endeavor that is subject to modification as new information challenges current theories. It Summative Assessme Design and conduct a sc the identity an unknown and defend to an audien investigation. Formative Assessmen Peer Practice- Ste Peer Practice – La Analyze quantitat Graph and interp Apply the design process by proposing solutions, testing, analyzing and modifying designs, and communicating results Propose solutions based upon understanding of properties of matter concepts Create designs Summative Assessme Apply the design process and communicate) to cre can be used to separate Analyze designs Communicate solutions Develop an explanation based upon evidence/data Report and display the process and results of a scientific investigation. Use quantitative data and representational methods to analyze measurements. Formative Assessmen Vocabulary asses mixture, solution Peer Practice – S See assessments above involves the collection of data, the use of logical reasoning, argumentation and the devising of hypotheses and explanations informed by evidence. Habits of Mind Scientists keep honest/unbiased, clear and accurate records, value hypotheses and understand that more than one explanation can be given for the same evidence. Argue the placement of an element on the periodic table based upon its chemical characteristics. Apply accepted practices of science. Generate, represent and analyze data Collect data Formative: Represent data Analyze phase change graph i Analyze data Generate phase change graph Scientists value the role of computation and estimation in their work. Scientists use a variety of tools to inform their observations. Scientists organize information using tables, graphs, diagrams and symbols. Scientists question claims based on vague attributions and are skeptical of arguments based on small data samples. Scientists embrace unexpected results. Vocabulary Tier Two: evidence, claim, support, argue, defend, analyze, justify, unknown Tier Three: mixtures, solutions, pure substances, acids, bases, reactivity, phases, physical property, chemical property, element, compound, atom, molecule, conservation of matter, periodic table, characteristic property, density, volume, mass, boiling point, melting point/freezing point, phase change graph