Practice Problems for Concepts Chemical Equilibrium
advertisement

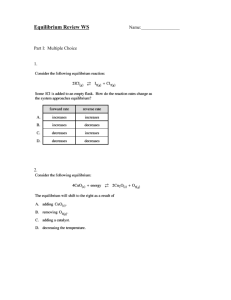
Practice Problems for Concepts Chemical Equilibrium through Nuclear Chemistry 1. The following reaction is at equilibrium: 4NH3 (g) + 3O2 (g) ↔ 6H2O (g) + 2N2 (g) How will the equilibrium shift if: a. The volume is increased b. Four moles of Helium gas is added c. A lit match is placed inside the container 2. This equation is at equilibrium: CO(g) + H2O (g) ↔ CO2 (g) + H2 (g) a. If a 10.00L vessel has 2.50 mol CO2 and H2O, and 5.00 mol CO2 and H2 gas at 588°K, which way will the reaction proceed? (Kc = 31.4 at 588°K) b. What are the concentrations of all species at equilibrium? 3. What is the pH of a solution containing 50.0mL of 1.00M HCl and 100.0mL of 0.25M HCl? 4. What is the pH of a 1.75M NH3 solution when it has reached equilibrium? (KB=1.8x10-5) 5. What is the general pH of water when the following salts are added? a. FeCl2 b. NaCl c. NaCH3CO2 6. After an all-night drinking binge, an MSU student pukes 300.0mL of liquid into his trash can. Assuming the puke is 20% HCl and the rest does not affects the pH, what is the pH of the solution resulting from the puke being neutralized with 0.50M NH3? (KB=1.8x10-5) 7. What is the pH of a buffer with 0.75 mol Hypochlorous Acid and 0.50 mol of its conjugate base in a 200.0mL solution? (KA = 3.5x10-8) 8. 500.0mL of a buffer with pH of 4.50 is required. If the acid is to be 0.80M, what mass of the lithium salt of the conjugate base is needed? Acid Formula KA Value Benzoic C6H5CO2H 6.3x10-5 Cyanic HOCN 3.5x10-4 Phenol C6H5OH 1.3x10-10 9. You have a 50.0mL solution of 1.35M NH3 which you are titrating with a 0.75M solution of HBr a. What is the pH before you start titration? b. What is the pH at the halfway point of titration? c. What is the pH at the equivalence point? d. What is the pH after adding 110.0mL of HBr? 10.The Ksp for lead(II) chloride is 1.7x10-5. If you place 3.00g of this solid in water, how much solid will be left after the system reaches equilibrium? (Hint: Find the molarity of the disassociated lead ion. It is equal to how much the molarity of the PbCl2 decreases by since they are in a 1:1 ratio) 11.How much of the above solid will be left if the solid is instead placed in a 0.01M HCl solution? 12.Feeling despair after the last chemistry exam, a Lyman Briggs student decides to instead try and strike it rich by retrieving precious metals from toxic waste water. If the water he is treating contains lead, barium, silver and sodium, what is the best way to obtain the silver and make the water drinkable? 13.What is the balanced red-ox reaction for the following reaction: C2O4–2 + MnO4 → CO2 + Mn+2 14.Given these two following half-reactions, which combination will most likely occur? (Hint: The one that will want to happen) Ni+2 + 2e– → Ni(s) ERed = -0.25V Cu+2 + 2e– → Cu(s) ERed = 0.153V 15.Given this reaction: CH4 (g) + 2O2 (g) → 2H2O(g) + CO2 (g) what are the ΔG values at standard conditions and at 477°C ? CH4 (g) 2O2 (g) 2H2O(g) CO2 (g) kJ Hf° ( /mol) -74.87 0 -241.83 -393.509 J S° ( /K*mol) 186.26 205.07 188.84 213.74 kJ Gf° ( /mol) -50.8 0 -288.59 -394.359 16.What is the temperature at which the above reaction changes between spontaneous and non-spontaneous? 17.What is the K value for the reaction in question 15 at 750°K? 18.What is the ΔG value for the resulting equation in question 14? 19.If you have a radioactive sample with a half life of 25 days, what percent will be left after 60 days? 20.What results when uranium-235 a. reacts with three neutrons? b. breaks into krypton and barium each with 20 extra neutrons? c. undergoes electron capture twice? Practice Problem Answers 1. The following reaction is at equilibrium: 4NH3 (g) + 3O2 (g) ↔ 6H2O (g) + 2N2 (g) How will the equilibrium shift if: a. The volume is increased Since the volume is being increased, the pressure is dropping. In response, the equilibrium will shift right to attempt to make more moles of gas and increase the pressure b. Four moles of Helium gas is added The equilibrium will not change. To change a gaseous equilibrium, the partial pressures of at least one gas need to be changed. Since He is un-reactive with all of the gases present, the total pressure will change but the partial pressures will not c. A lit match is placed inside the container The match is a combustion reaction and requires O2 to proceed. Thus, the oxygen in the system will be used and the equilibrium will shift left in order to compensate for the loss. 2. This equation is at equilibrium: CO(g) + H2O (g) ↔ CO2 (g) + H2 (g) d. If a 10.00L vessel has 2.50 mol CO2 and H2O, and 5.00 mol CO2 and H2 gas at 588°K, which way will the reaction proceed? (Kc = 31.4 at 588°K) Since Qc < Kc, it will proceed in the forward direction a. What are the concentrations of all species at equilibrium? [CO] & [H2O] = 0.114M while [CO2] & [H2] = 0.636M 3. What is the pH of a solution containing 50.0mL of 1.00M HCl and 100.0mL of 0.25M HCl? pH = 0.30 4. What is the pH of a 1.75M NH3 solution when it has reached equilibrium? (KB=1.8x10-5) pH = 11.75 5. What is the general pH of water when the following salts are added? b. FeCl2 Fe+2 is a transition metal while lends acidity to the solution. Clis the conjugate of a strong acid so it is non-basic. Therefore, the solution will be acidic c. NaCl Na is an alkali metal so it does not affect the pH. Cl- does not affect the pH for the reasons above, so the solution is neutral d. NaCH3CO2 Na has no effect (see above) while CH3CO2 is the conjugate base of a weak acid so it is somewhat basic. The overall solution is then basic. 6. After an all-night drinking binge, an MSU student pukes 300.0mL of liquid into his trash can. Assuming the puke is 20% .10M HCl and the rest does not affects the pH, what is the pH of a the solution resulting from the puke being neutralized with 0.50M NH3? (KB=1.8x10-5) pH = 5.15 7. What is the pH of a buffer with 0.75 mol Hypochlorous Acid and 0.50 mol of its conjugate base in a 200.0mL solution? (KA = 3.5x10-8) pH = 7.28 8. 500.0mL of a buffer with pH of 4.50 is required. If the acid is to be 0.80M, what mass of the lithium salt of the conjugate base is needed? Acid Formula KA Value Benzoic C6H5CO2H 6.3x10-5 Cyanic HOCN 3.5x10-4 Phenol C6H5OH 1.3x10-10 102.45g of the lithium salt is needed 9. You have a 50.0mL solution of 1.35M NH3 which you are titrating with a 0.75M solution of HBr (KB (NH3) = 1.8x10-5) e. What is the pH before you start titration? pH = 10.19 f. What is the pH at halfway to the equivalence point? Note that the equivalence point is where the number of moles base you have first equals zero. So halfway to this point means half of your base remains and the other half has been converted to conjugate acid. This results in having a buffer where the ratio of moles base to moles conjugate acid is 1. Since log(1) = 0, the pH at this point is just the pKA. pH = 9.22 g. What is the pH at the equivalence point? pH = 4.77 h. What is the pH after adding 110.0mL of HBr? The volume of HBr added here is greater than the amount added at the equivalence point so HBr is in excess and the pH is equal to –log [HBr] pH = 0.125 10.The Ksp for lead(II) chloride is 1.7x10-5. If you place 3.00g of this solid in 100.0mL water, how much solid will be left after the system reaches equilibrium? (Hint: Find the molarity of the disassociated lead ion. It is equal to how much the molarity of the PbCl 2 decreases by since they are in a 1:1 ratio) No precipitate is left 11.How much of the above solid will be left if the solid is instead placed in a 0.05M HCl solution? 0.100g of precipitate is left 12.Feeling despair after the last chemistry exam, a Lyman Briggs student decides to instead try and strike it rich by retrieving precious metals from toxic waste water. If the water he is treating contains barium, strontium, silver and calcium, what is the best way to obtain the silver and make the water drinkable? There are several ways to solve this problem so here is one example: To precipitate the strontium and barium only, add SO4–2. Then add F– to precipitate the silver. Calcium in water is fine, so he is done. 13.What is the balanced red-ox reaction for the following reaction: C2O4–2 + MnO4 → CO2 + Mn+2 14.Given these two following half-reactions, which combination will most likely occur? (Hint: The one that will want to happen) Ni+2 + 2e– → Ni(s) ERed = -0.25V Cu+2 + 2e– → Cu(s) ERed = 0.153V Since Cell #2’s Ecell is positive, it is the most likely to occur 15.Given this reaction: CH4 (g) + 2O2 (g) → 2H2O(g) + CO2 (g) what are the ΔG values at standard conditions and at 477°C ? CH4 (g) 2O2 (g) 2H2O(g) CO2 (g) kJ Hf° ( /mol) -74.87 0 -241.83 -393.509 J S° ( /K*mol) 186.26 205.07 188.84 213.74 kJ Gf° ( /mol) -50.8 0 -288.59 -394.359 Standard Conditions: -920.74 KJ At 477°C: -798.564 KJ 16.What is the temperature (K) at which the above reaction changes between spontaneous and non-spontaneous? Tc = 161,104°K 17.What is the K value for the reaction in question 15 at 750°K? K = 1.14 18.What is the ΔG value for the most likely cell in question 14? ΔG = -77779 KJ 19.If you have a radioactive sample with a half life of 25 days, what percent will be left after 60 days? About 19% of the sample is left 20.What results when uranium-235 Uranium-235 is the same thing as saying 235 92 U . Also keep in mind that the element changes only if the bottom number (the atomic number, represented by Z) changes a. reacts with three neutrons? This results in uranium-238 b. breaks into krypton and barium each with 20 extra neutrons? We know that the atomic numbers for krypton and barium are 36 and 56 respectively so the normal number of neutrons for each are 72 and 112. Since they each have 20 extra neutrons, their A values are now 92 and 132. We now know the equation looks something like this: 92 U 36 Kr 132 56 Ba 235 92 36+56 is 92 so we are set in terms of protons and electrons. However, 235–92–112 results in 11 so that’s how many neutrons need to be added. The final equation is: 92 1 U 36 Kr 132 56 Ba 110 n 235 92 c. undergoes electron capture twice?


![CHEM 1520 SI MON, TUES, & WEDNES 1.Calculate [H3O+] in a](http://s3.studylib.net/store/data/007346334_1-b78d73402f58153c92290299886ff084-300x300.png)