Supplementary Material (doc 63K)
advertisement

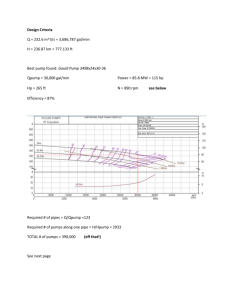
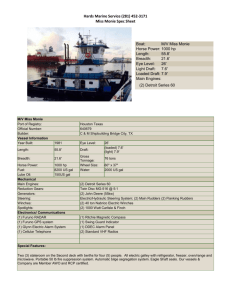
Chu et al. Supplemental Supplementary Material Materials & Methods Preparation of pGZCUBI-HAGA The construction of the CpG-reduced pGZCUBI-HAGA vector, in which a ubiquitin B promoter drives the expression of -galactosidase A, has been described [1]. Schematic diagrams of plasmids used in these studies Figure 1 depicts schematically the two plasmids used for the studies described in the main text, namely, pCMV and pHRP. Results - Pilot Experiments Hydrodynamic delivery of -galactosidase A elicits a humoral response. Hydrodynamic delivery of naked pDNA to the liver represents a potential approach by which secreted therapeutic proteins can be produced from a liver “depot”. Fabry disease is a lysosomal storage disease, the gene for which, -galactosidase A (gal), is mutated in Fabry patients. For comparative purposes, we have conducted parallel experiments in both the BALB/c and Fabry mouse strains. In principle, the BALB/c strain should represent a relatively “low” immunologic hurdle, since it produces an endogenous version of gal. By contrast, the Fabry mouse might be expected to present a significantly higher set of immunologic hurdles - it is immune competent, produces no active protein [2] and may therefore recognize any gal produced as “foreign”. As an initial attempt to explore this question, a pilot experiment was designed to compare the results of hydrodynamic delivery of a plasmid bearing gal in these two mouse strains. Chu et al. Supplemental Figure 2A shows that hydrodynamic delivery of two different doses of a CMV-driven plasmid bearing the synthetic human -galactosidase A gene (pCMV) to BALB/c mice resulted in an expression profile characterized by an initial rapid loss of serum levels of gal (~1 log in 3 weeks) followed by prolonged serum levels of ~1 µg/ml. In contrast to these results in the BALB/c mouse, Figure 2B shows that in the Fabry mouse strain, which is a transgenic knockout for gal, expression declined much more dramatically, so that at the 3 week post-administration time point, serum levels were <30 ng/ml; serum levels continued to decrease significantly over the next several months. Consistent with the more rapid loss of serum gal in the Fabry mouse was the more rapid appearance of anti-gal antibodies. Thus, in the BALB/c mouse, no antibodies were observed until day 84 (data not shown), and Figure 2C shows that the humoral response was relatively minimal even at day 112. In contrast, Figure 2D shows that in the Fabry mouse, detectable antibody levels, i.e., titers ≥200, were observed at day 42 post-administration in some animals, even at the lower pCMV dose. Moreover, for both BALB/c and Fabry strains, the onset of detectable serum antibody levels appeared to correlate with initial serum levels of gal. For example, in the BALB/c mouse, detectable antibody titers were present by day 84 only in the high dose (10 µg) group (data not shown); significant titers were apparent in the low dose (0.5 µg) group only after day 112 (Fig 2C). By contrast, in the Fabry mouse, a larger fraction of mice had detectable antibody titers in the high dose group than the low dose group at both day 42 and 112 (Fig 2D). Taken together, the results from this pilot study suggest that hydrodynamic delivery of an gal plasmid can elicit a humoral response against the gene product, that this Chu et al. Supplemental response is more pronounced in the knockout (Fabry) model, and that it may correlate with initial expression levels. Hydrodynamic delivery of an gal plasmid does not appear to elicit a cellular immune response against gal. To ask whether cytotoxic T lymphocytes (CTLs) played a role in the declining serum levels of gal observed in Figure 2, a pilot experiment was conducted in which both liver and serum levels of gal were measured over time. Figure 3A demonstrates that liver expression of gal continues for at least 6 weeks in the face of declining serum levels of the expressed transgene. Figure 3B shows that the humoral response to gal increases over this same time period. Thus, this pilot study suggests that CTLs do not contribute significantly to the time-dependent changes in gal levels seen after hydrodynamic delivery of gal plasmids to the liver. CAT-siRNA specifically inhibits CAT expression in mouse liver. Our strategy involves siRNA-mediated inhibition of the expression of a gene delivered to the liver by hydrodynamic injection. To characterize the time-dependent effects of siRNA on the expression of a model exogenous gene from mammalian liver, plasmid DNA bearing the reporter gene chloramphenicol acetyltransferase (CAT) was administered to mice together with siRNA using the hydrodynamic procedure. Figure 4A demonstrates that 1 day after this co- administration, the cognate siRNA, namely CAT-siRNA, significantly attenuated CAT expression in the liver. In quantitative terms, co-administration of 0.5 µg CAT-siRNA and 1 µg of pCF1-CAT resulted in an approximately 70% decrease in CAT expression at 1 day after delivery (compared to no siRNA). This decrease was apparently maximal, since a ten-fold Chu et al. Supplemental increase in the amount of CAT-siRNA co-administered, viz., 5 µg CAT-siRNA with 1 µg pCF1CAT, resulted in essentially the same decrease in expression (data not shown). The ability of the co-administered CAT-siRNA to decrease CAT expression was apparently sequence specific, since 5 µg of an siRNA bearing an inverted sequence (CAT-inv-siRNA) failed to change CAT expression significantly. Figure 4B also demonstrates that the CATsiRNA was specific for CAT in that at the same dose (5 µg) it had essentially no effect on the expression from 1 µg of the unrelated reporter gene SEAP. It is also worth noting that the attenuation of CAT expression seen here could not be duplicated with equivalent (mass) amounts of CAT-specific sense or anti-sense (single-stranded) oligonucleotides derived from the CATsiRNA, i.e., no significant decrease in CAT expression 1 day after co-administration (Fig 4A). The inhibition of CAT expression in the liver by a co-administered cognate siRNA was found to be relatively long-lived. In another set of experiments, 1 day after the co-administration of 2 µg pCF1-CAT together with 1 µg CAT-siRNA, CAT expression in the liver was decreased by 71% (±12%; data not shown). Over the two week duration of the experiment, CAT expression increased only slowly, i.e. it was 52±16% relative to pCF1-CAT alone (data not shown). Thus, these data indicated that the siRNA-mediated suppression of expression was relatively longlasting, at least for the CAT reporter. siRNA inhibits expression of -galactosidase A from mouse liver. To ask whether an siRNA-based strategy could also decrease liver expression of a normal mammalian gene, we used gal, the enzyme deficient in Fabry disease. Figure 4C shows that one day after hydrodynamically delivering 10 µg of a plasmid bearing the cDNA for synthetic Chu et al. Supplemental human gal (pCMV) to BALB/c mice, serum gal levels were ~5800 ng/ml; serum gal levels in naive controls were <0.01 ng/ml (data not shown). Figure 4C shows that co-administering 10 µg of an gal-siRNA decreased the serum gal level by >98%. Figure 4C also shows that these decreases effected by the gal-siRNA were specific for the targeted gene, namely gal, since the same 10 µg dose of the above CAT-siRNA (Fig 4 A,B) had no significant effect on gal expression. Figure 4D demonstrates that this gal-siRNA mediated effect was dose-dependent, viz., in an independent experiment, 1 and 10 µg of co-administered gal-siRNA decreased serum gal levels by 94 and 98%, respectively. Taken together, the data in Figure 4 demonstrate that co-delivering to the liver a plasmid bearing a gene for either a reporter or endogenous protein (CAT or gal), together with the siRNA specific for that gene can result in rapid and significant decreases in expression of the corresponding protein. Hydrodynamic delivery elicits an inflammatory response in mice. We have noted previously that hydrodynamic delivery in rabbits elicits an inflammatory response that can be characterized by serum levels of IL-12 and the serum transaminases, eg. alanine aminotransferase, ALT [3]. In the current study, 18 h after injecting pCMV in BALB/c mice ALT levels had increased from baseline levels (24 ±11 IU/L) to 2230 ±224 IU/L), and IL12 had increased from baseline (94 ±32 pg/ml) to 180 ±9 pg/ml. We also asked whether coadministering an siRNA exerted a significant effect on these toxicity measures. At the same 18 h time point, serum ALT levels for BALB/c mice treated with pCMV and CAT-siRNA or galsiRNA were 2044 ±656 and 1782 ±473 IU/L, respectively. These levels were not significantly different from those found from mice treated with pCMV alone (see above). Serum IL-12 levels were also not significantly different for mice administered pCMV alone (180 ±9 pg/ml), or Chu et al. Supplemental pCMV together with CAT-siRNA (160 ±10 pg/ml) or gal-siRNA (140 ±19 pg/ml). Similar results were observed with pHRP, and also with Fabry mice (data not shown). Thus, delivering an siRNA together with pDNA resulted in ALT and IL-12 levels that were not significantly different from hydrodynamic delivery of the pDNA alone, implying that at these doses there is no significant additional toxicity attributable to the siRNA. Chu et al. Supplemental References 1. Yew NS, Przybylska M, Ziegler R, Liu D, and Cheng SH. (2001). High and sustained transgene expression in vivo from plasmid vectors containing a hybrid ubiquitin promoter. Mol Ther. 4:75-82. 2. Ioannou YA, Zeidner KM, Gordon RE and Desnick RJ. (2001). Fabry disease: preclinical studies demonstrate the effectiveness of alpha-galactosidase A replacement in enzymedeficient mice. Am J Hum Genet. 68: 14-25. 3. Eastman SJ, Baskin KM, Hodges BL, Chu Q, Gates A, Dreusicke R, Anderson S, and Scheule RK. (2002). Development of catheter-based procedures for transducing the isolated rabbit liver with plasmid DNA. Hum Gene Ther. 13:2065-2077. Chu et al. Supplemental Figure Legends Figure 1. Schematic diagrams of human gal plasmids compared in these studies. The ubiquitously-expressed pGZB-sHAGA plasmid is abbreviated in the text as “pCMV”, and is composed of a synthetic, CpG-free CMV (sCMV) promoter, hybrid intron (sHI), human gal cDNA (sHAGA), and bovine growth hormone (sBGH) poly A, a CpG-reduced and minimal bacterial origin of replication (Ori), and a synthetic CpG-free kanamycin resistance gene (sKan). The hepatocyte-restricted pGZDC190-sHAGA plasmid, which is abbreviated in the text as “pHRP”, contains these same elements, but it is driven by the DC190 promoter, which is composed of a human serum albumin promoter to which are appended two copies of the human prothrombin enhancer. Figure 2. Hydrodynamic delivery of synthetic human gal plasmids generates variable serum gal levels and anti-gal antibodies in BALB/c and Fabry mice. BALB/c (A & C) and Fabry (B & D) mice were hydrodynamically injected with either 10 µg (open circles) or 0.5 µg (filled circles) of pGZB-sHAGA (pCMV). Serum levels of gal (A & B) and anti-gal antibodies (C & D) were determined by ELISA (see Methods). Symbols in A and B represent mean levels of gal ±SD; in C and D, each animal is represented by the same symbol (filled symbol 0.5 µg, open symbol 10 µg) at both time points; titers<200 are considered negative (shaded area). N=56 animals per group. Figure 3. Kinetics of -galactosidase A expression from pGZCUBI-HAGA. Using hydrodynamic delivery, 10 µg of pGZCUBI-HAGA was injected into Fabry mice. Groups of 5 Chu et al. Supplemental mice were killed at each time point and liver tissue and serum assayed for gal by ELISA (A). See Methods for ELISA of serum; analysis of liver homogenates was as previously published [1]. Anti-gal antibody titers in serum were determined (see Methods) at each time point (B). Shaded area in (B) represents titers considered negative. Figure 4. CAT-siRNA specifically inhibits CAT expression in mouse liver and an gal-siRNA provides specific, dose-dependent inhibition of gal expression in BALB/c mice. (A) A pDNA (1 µg) bearing CAT (pCF1-CAT) was hydrodynamically injected into BALB/c mice either alone or together with a CAT-siRNA (0.5 µg) or one of several control RNA constructs (5 µg), and CAT expression in liver homogenates determined 1 day later. Bars represent means ± SD. N=5 animals per group. (B) A pDNA (1 µg) bearing an sSEAP expression cassette (pGZB-sSEAP) was hydrodynamically injected either alone or with 5 µg of the same CAT-siRNA used in (A). SEAP expression was determined in serum 1 day later. Bars represent means ± SD. N=5 animals per group. (C) A pDNA (10 µg) bearing human gal (pCMV) was injected either alone or together with 10 µg of a CAT-siRNA or an gal-siRNA. Serum expression of gal was determined 1 day later. Bars represent means, which are also shown numerically; error bars represent SD. N=14 animals/group. The p value shown is relative to the pCMV group. (D) A pDNA (10 µg) bearing human gal (pCMV) was injected either alone or together with 1 or 10 µg of an gal-siRNA. Serum expression of gal was determined 1 day later. Bars represent means which are also shown numerically; error bars represent SD. N=14 animals/group. The p value shown is relative to the pCMV group.