The pH of acids at different concentrations - scie
advertisement

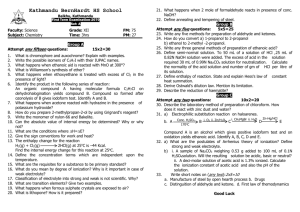
The pH of acids at different concentrations Aims 1 2 To compare a strong and a weak acid To examine the effect of dilution on a weak acid Introduction Hydrochloric acid is a strong acid which ionises completely in water: HCl(g)+ H2O(l) + H3O (aq) + Cl -- (aq) Ethanoic acid is a weak acid which ionises only partially in water: CH3COOH(l) + H2O(l) = H3O+(aq) + CH3COO-(aq) The extent of ionisation depends on the initial concentration of the ethanoic acid. By measuring the pH at different concentrations, you can see the effect of dilution. These results can be generalised for any weak acid. Requirements pH meter (already calibrated), distilled water, 2 x 10 cm3 pipettes, pipette filler, 2 x 100 cm3 volumetric flasks, 8 x 100 cm3 beakers, 0.10M hydrochloric acid, 0.10M ethanoic acid Procedure 1 Pipette 10 cm3 of 0.10M hydrochloric acid into a 100 cm3 volumetric flask. Make up to the mark with distilled water, insert the stopper and shake well for at least a minute. Transfer this solution into a clean, labelled beaker. This solution has a concentration of 0.010M. In a similar way, use the method of successive dilutions to prepare 0.0010M and 0.00010M solutions of hydrochloric acid. Starting with the least concentrated solution, find the pH of each solution using a pH meter. 2 Repeat step 1 starting with 0.10M ethanoic acid. 3 Calculate the expected pH values of each solution of hydrochloric acid Results Concentration of acid/mol dm-3 Calculated pH of solutions of hydrochloric acid Measured pH of solutions of hydrochloric acid Measured pH of solutions of ethanoic acid 0.00010 0.0010 0.010 0.10 Questions 1 Compare the pH of ethanoic acid with hydrochloric acid at each concentration. (i) In which of the two acids is the concentration of hydrogen ions greater? (ii) What does this tell you about the extent of ionisation of ethanoic acid compared with hydrochloric acid? 2 (i) What happens to the difference between the pH of the two acids as concentration decreases? What does this tell you about the effect of dilution on ionisation? (ii) Use Le Chatelier’s Principle to explain the effect of dilution on the extent of ionisation of ethanoic acid. MARKING Results table completed (1) pH decreases as acid concentration decreases (1) Q1 (i) [H+] greater in hydrochloric acid[1] (ii) Extent of ionization less in ethanoic acid [1] Q2 (i) pH decreases as acid concentration decreased [1] Degree of ionization of ethanoic acid increases on dilution [1] (ii) CH3COOH + H2O => CH3COO+ H3O+ + Ka = [CH3COO ][ H3O ]/[ CH3COOH] Addition of water causes [CH3COO-], [ H3O+] and [ CH3COOH] to all decrease. Because [CH3COO-] and [ H3O+] are on the rhs and only [CH3COOH] is on the lhs, a shift to the right goes further towards restoring the original concentrations than a shift to the left. [4] Total 10 marks