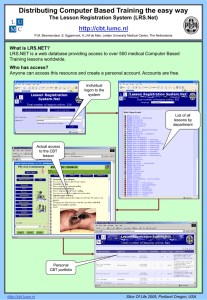
Project Key: LRS/06/11/050
advertisement

Minutes – November Meeting File RE08-04-4 Page – 1 LOWER SOUTH REGIONAL ETHICS COMMITTEE MEETING HELD ON TUESDAY 7 NOVEMBER 2006 MEETING ROOM L4.4 FOURTH FLOOR, MINISTRY OF HEALTH 229 Moray Place, Dunedin at 1.15 p.m. MEMBERS PRESENT: Mrs. Jenny Beck (Chair) Dr. Phil White (Deputy Chair) Mrs. Fay McDonald Mr. Kenneth Copland Dr. Gail Tripp Mrs. Gwen Neave Dr. Rosemary Beresford Dr. Nikki Kerruish Mrs. Sandra Elkin Dr. Alan Payne Dr. Khyla Russell APOLOGIES: Dr. Clare Robertson IN ATTENDANCE: Riria Tautau-Grant Carolyn Mason LRS/06/11/050 LRS/06/11/051 LRS/06/11/052 LRS/06/11/057 LRS/06/11/054 LRS/06/11/055 LRS/06/11/056 Dr. Rob MacGinley, Dr. John Schollum, Professor Stephen Duffill and Alwyn Todd Rajiv Verma and Dr Warwick Duncan Professor Robin Taylor Dr. Douglas Cowan Administrator Chairperson Upper South A Co-Researchers 1.45 p.m. Co-Researchers Co-Researchers 2.30 p.m. 3.45 p.m. MINUTES OF THE PREVIOUS MEETING - The minutes of the closed meeting and open meeting held 3 October 2006 were confirmed. Minutes – November Meeting File RE08-04-4 Page – 2 NEW PROTOCOLS 1. PROJECT KEY: LRS/06/11/049 Full Title: What is the incidence and awareness of Asthma in the Southland Region Investigators: Jill Parsons, Lesley Watson, Ed Flagg, Cara Agnew Localities: Participant's homes, Southern Institute of Technology DEFERRED Ethical approval was deferred pending substantial revision of the proposal and satisfactory answers to the questions being asked. Note: Provide a copy of the Top Ten Tips A VALIDITY OF RESEARCH - Different people are likely to be home at different times of the day, is the timing of the calls being made to participants, going to bias the sample? B MINIMISATION OF HARM - B 13 Please clarify whether the researchers will be present when all the calls are made - What is the process if additional health professionals input is required? - It is noted that the Bachelor of Nursing students participation in this research is significant. Please provide a description of what educational value will be gained by participating nursing students. The Committee would prefer that: (a) no coercion is involved in having the nursing students participate (b) that they are remunerated appropriately D PRIVACY AND CONFIDENTIALITY - D6 Raw data needs to be kept for 10 years if it includes health information and according to Institute’s policies if it does not include health information - D7 This is health information and must be kept for 10 years. Please ensure uniformity throughout the documentation E INFORMED CONSENT - Please describe how you plan to manage the issue of consent for and access to those under 16 years old: - Please note that: (a) Some of those under 16years old will be able to give consent and take part in the survey; and (b) Those who are not taking part in the survey but about whom information is being sought should be allowed to assent or not. See Operational Standards for Ethics Committees, Appendix 1, Pg. 54 Para. 263. F CULTURAL AND SOCIAL RESPONSIBILITY - F3.1 – please revisit this question as the answer is likely to be “Yes” - Consultation with Māori is required. Please forward evidence of consultation. LOCALITY ASSESSMENT - A locality assessment is required from the Southern Institute of Technology APPENDIX 1 NEWSPAPER ADVERTISEMENT / INFORMATION SHEET - Pg. 13 Para 4 Please use the full name of the Committee as follows: the Lower South Regional Ethics Committee Minutes – November Meeting File RE08-04-4 Page – 3 - - - Pg. 13 Para 5 Please replace the last four words of this paragraph with a full stop after the word team Pg. 14 sect 6 The last paragraph mentions a follow up study. The Committee needs to know what is involved in the follow up study, and wishes it noted that full approval of this study does not establish ethical approval for the follow up study. Pg. 14 sect 6 Please change the last sentence to read as follows: “ If you supply your name and contact details for the follow up study, that information will be kept by the research team only, then destroyed at the completion of that study” More details regarding the “further study” should also be provided in the information sheet. Please provide a copy of the approval given by the Southern Institute of Technology Research Committee APPENDIX 2 QUESTIONNAIRE - The ethnicity question is not appropriate. Please use the ethnicity question from the census - Q 14 Add “other” as a choice - Q 15 Please explain what is meant by an Asthma management plan. The terminology may not be familiar to everyone. - Q 25 As stated above, the Committee needs to see information about a follow up study APPENDIX 4 LETTER FROM SOUTHLAND ASTHMA SOCIETY INC. - See also A7.1. The Committee has chosen to defer this study. Will the funding for the project be still available post ethical approval? APPENDIX 5 PROCEDURAL STEPS - Add an instruction to ask “Is this a good time to call?” - No. 4 Is it appropriate to include a question about whether another member of the household has asthma? so that they can be interviewed about their asthma. 2. PROJECT KEY: LRS/06/11/050 Full Title: An audit of prescribing patterns in patients who are obese Investigators: Professor Stephen Duffull, Dr. Ruth Ferguson, Dr. John Schollum Localities: School of Pharmacy, University of Otago, Otago District Health Board APPROVED SUBJECT TO CONDITIONS Note: Provide a copy of the Top Ten Tips - Dr. Rob MacGinley, Alwyn Todd, Dr. John Schollum and Professor. Stephen Duffill attended the meeting. PART 1 : BASIC INFORMATION - No. 6 Please provide information about the summer student. Include their background and training. What will they do if they know the participant? D PRIVACY AND CONFIDENTIALITY - Please provide information regarding the procedures under which the student will safeguard the privacy of participants and of health and personal information. F CULTURAL AND SOCIAL RESPONSIBILITY Minutes – November Meeting File RE08-04-4 Page – 4 - Please provide a copy of the letter evidencing that consultation has been completed. CONSENT FORM - It is noted that If the height and weight is recorded on the form that there will be no need to gather consent LOCALITY ASSESSMENT - A locality assessment from is required for the ODHB 3. PROJECT KEY: LRS/06/11/051 Full Title: Enoxaparin and the risk of bleeding - an impact study Investigators: Professor Stephen Duffill, Hesham Al-Sallami, Ruth Ferguson, Natalie Medlicot, Dr. John Schollum Localities: Dunedin Hospital, School of Pharmacy APPROVED SUBJECT TO CONDITIONS - Dr. Rob MacGinley, Alwyn Todd, Dr. John Schollum and Professor. Stephen Duffill attended the meeting. BASIC INFORMATION - 18-21 – Please clarify timeline specifically in relation to A4.1 B MINIMISATION OF HARM - B10 Explain why patients less than 18 years old are being excluded C COMPENSATION FOR HARM - C As health professionals are involved in the assessment of participants, ACC would regard this as a clinical trial. The answer should be “Yes” Please forward a signed copy of the Form A F CULTURAL AND SOCIAL RESPONSIBILITY - F3.2 Please forward a copy of the letter from Ngai Tahu once that is received - F3.3 Please modify the response here as it contradicts the response at F3.2. - F3.4 Explain why it was decided that ongoing involvement with groups consulted is not applicable. The Committee notes that there are a number of issues involving blood that raise cultural considerations for Maori. INFORMATION SHEET - Provide information sheet on a letterhead - The Committee commends the good use of language - Increase the font size for the name and number for the Principal investigator, and place into the body of the information sheet. - Please place all investigators names on this sheet - Pg. 2 Para 10 Omit the words ‘for New Zealand’ - Pg. 2 Para 11 Please add the word “be” between “…individual…” and “…released…” - ACC is mentioned on pg.1 ‘what are side effects’ and pg. 2 ‘Compensation.’ The second mention is sufficient - Please use preferred abbreviated ACC statement. Refer to Top 10 Tips document appended. - The Health and Disability advocacy statement has been recently updated. The new statement is included in the Top 10 Tips document appended. Minutes – November Meeting File RE08-04-4 Page – 5 CONSENT FORM - No. 6 Remove the question to notify GP’s of adverse events LOCALITY ASSESSMENT - Please provide locality assessment forms for the ODHB and the School of Pharmacy HUMAN TISSUE - 1.7 Describe the protocol for transferring blood from the Hospital to the School of Pharmacy FORM A - Please provide a copy of the Form A 4. PROJECT KEY: LRS/06/11/052 Full Title: A randomised single blind study to improve health related quality of life as measured by the SF-36 vitality score by correcting anaemia of chronic kidney related disease with Aranesp® (darbepoetin alfa) in the elderly (STIMULATE) Investigators: Dr. John Schollum, Professor Rob.Walker, Dr. Rob. MacGinley, Gaye Ellis Localities: Department of Medical and Surgical Sciences APPROVED SUBJECT TO CONDITIONS - Dr. Rob MacGinley, Alwyn Todd, Dr. John Schollum and Professor. Stephen Duffill attended the meeting. C COMPENSATION FOR HARM - Please complete a Form B - ACC compensation provisions are baseline. The Company appear to be offering less than that which ACC would usually provide. Please discuss with the company the need to provide at least what is covered by ACC. A copy of the Health and Disability Ethics Committees guidelines are appended. F CULTURAL AND SOCIAL RESPONSIBILITY - F4 Please revisit this question acknowledging the comments of the Ngai Tahu Research Committee INFORMATION SHEET - The Committee notes that there are imposed restrictions on investigator’s ability to reconfigure the information sheet: (a) Where possible please make the following changes to the information sheet. (b) Where it is not possible, then the Committee request that the information be provided in a summary/ guide: 2. Pg. 5 Para 5 Link paragraphs 3(a) and 3(b) to make it clearer that the second paragraph describes the ‘associated side effects’ 3. Simplify the language 4. Use the term participants rather than subjects 5. Provide more information about the background drug and how long it has been available 6. Pg. 3 Q 3b Make it clearer that if people in the placebo group really Minutes – November Meeting File RE08-04-4 Page – 6 need the drug, that they will get it. 7. Add the right to withdraw earlier in the information sheet, with the background information 8. Make it clear that Amgen makes and owns the license for the drug REGISTERED DRUG FORM - The committee would like to know the chemical constituents of the clear colourless fluid being used as placebo LOCALITY ASSESSMENT - Please forward a copy of this as soon as possible 5. Project Key: LRS/06/11/053 Full Title: Double blind randomised controlled trial of an orally administered probiotic in the treatment of spondylo arthritis Investigators: Dr. Simon Stebbings, Dr. Michael Schultz, Professor John Highton Localities: Rheumatology Outpatient Dept., Microbiology Dept, Chemistry Dept. APPROVED SUBJECT TO CONDITIONS A VALIDITY OF RESEARCH - A3.1 Clarify whether you will require presence of HLA-B27 gene as a gateway onto the study B MINIMISATION OF HARM - B10 What is the reason for excluding persons with major depressive illnesses? If there is a likelihood of psychiatric distress, then this should be advised in the information sheet C COMPENSATION FOR HARM - Please complete and forward the Form A document F CULTURAL AND SOCIAL RESPONSIBILITY - F3.1 Please provide a copy of the letter form NTRCC as evidence of consultation - F3.3 Please clarify the consultation process undertaken, the Committee is aware that Christine Rimene has not held the liaison role for some time now. INFORMATION SHEET - Please apply a footer with a version no. and date - Number the pages - Pg. 3 Para 3 Simplify the language used, clearly explaining the implications - Pg. 3 Para 7 An “or” is missing. Please insert “or” in the second line, between “…group…” and “…the placebo…” - Please explain where the analysis of the samples will take place, at the Dept of Pharmacology as per the information sheet or at the Dept of Chemistry as per the response at Basic Information no. 10 - Counselling needs to be mentioned, as it is offered at B12 of the application form - Pg. 3 Para 4 Replace ‘tests’ with ‘test’ - Pg. 3 Para 5 Replace ‘he’ with ‘the’ - Pg. 2 Para 6 Remove the word ‘an’ - Pg. 2 Para 6 Replace the first instance of ‘these’ with ‘this’ - Pg. 3 Para 6 ‘labelled’ is spelt incorrectly - Pg. 3 Para 6 Use ‘number’ instead of no Minutes – November Meeting File RE08-04-4 Page – 7 - The Health and Disability statement has been updated. Please refer to Top 10 Tips appended CONSENT FORM - Add a consent question for genetic testing LOCALITY ASSESSMENT - Please forward completed locality assessment forms from the ODHB and the University of Otago 6. PROJECT KEY: LRS/06/11/054 Full Title: Retrospective analysis of dental implant treatment provided at the School of Dentistry since 1989 Investigators: Rajiv Verma, Dr. Warwick Duncan, Ms. Mary Cullinan, Professor Gregory Seymour Localities: School of Dentistry, University of Otago APPROVED SUBJECT TO CONDITIONS - Rajiv Verma and Dr. Warwick Duncan attended the meeting B MINIMISATION OF HARM - The committee is concerned that every possible measure should be taken to avoid contact with the families of people who have died since their original treatment. C COMPENSATION FOR HARM - C Please answer yes. ACC would regard this as a clinical trial as assessment and treatment is being undertaken by a health professional. Form A is required F CULTURAL AND SOCIAL RESPONSIBILITY - F3 This needs to be answered in the affirmative - F3.1 Bullet point 3 Please revisit the response. The head is tapū, and this has some cultural considerations - F3.1 Bullet point 5 Please change “Whānau members are…” to “A whānau member is …” - Please take into account Mark Brunton’s recommendations INFORMATION SHEET - Pg.1 Para 1 Add that the two appointments will be for one hour each - This sheet contains two advocacy statements, only the first of which is correct. Replace the 2nd one on Pg.2 with the first one on page one. - Add the standard abbreviated ACC statement. This is appended in the Top 10 Tips sheet - It is understood that the exams and x-rays will be at no cost. Please make it clearer on pg. 3 para. 2 that treatments thereafter will be at usual costs CONSENT FORM - Add a consent for later use of plaque samples and add that later use will only occur providing ethical approval to do so is granted. RADIATION FORM - Please urgently complete a radiation form Minutes – November Meeting File RE08-04-4 Page – 8 7. PROJECT KEY: LRS/06/11/055 Full Title: Does obesity influence the pattern of lung function during acute bronchoconstriction? (the INCA study - Investigating broncho-constriction in asthma) Investigators: Professor Robin Taylor, Dr. Tim Sutherland, Mrs. Jan Cowan. Localities: Otago Respiratory Research Unit APPROVED SUBJECT TO CONDITIONS - Professor Robin Taylor and Dr. Douglas Cowan attended the meeting F CULTURAL AND SOCIAL RESPONSIBILITY - Please provide letter and feedback from Māori consultation INCA STUDY INFORMATION SHEET - Pg. 1 Para 4 Under Visit 1, bullet point 2 please remove the word ‘to’ - The H&D advocacy statement has recently been updated to reflect the additional contact services that they provide. A copy of that statement may be found in the appended Top 10 Tips. LOCALITY ASSESSMENT - Please provide a signed copy of the locality assessment forms for the University of Otago and the ODHB. REGISTERED DRUG FORMS - Please provide registered drug forms for methacholine and Ventolin 8. PROJECT KEY: LRS/06/11/056 Full Title: The management of non-eosinophilic asthma: a randomised controlled trial Investigators: Professor Robin Taylor, Dr. Douglas Cowan, Mrs. Jan Cowan. Localities: Otago Respiratory Research Unit APPROVED SUBJECT TO CONDITIONS - Professor Robin Taylor and Dr. Douglas Cowan attended the meeting F CULTURAL AND SOCIAL RESPONSIBILITY - F3.1 Please provide the response from the Ngai Tahu Research Consultation Committee - F3.5 Make the results known to Ngai Tahu Research Consultation Committee INFORMATION SHEET / CONSENT FORM - Use the term participants rather than subjects - The H&D advocacy statement has recently been updated to reflect the additional contact services that they provide. A copy of that statement may be found in the appended Top 10 Tips. AMP INFORMATION SHEET - Para 3 Please update this with correct details for researchers/respiratory dept. LOCALITY ASSESSMENT - Please provide completed locality assessment forms for the University of Otago and the ODHB Minutes – November Meeting File RE08-04-4 Page – 9 9. PROJECT KEY: LRS/06/11/057 Full Title: The impact of dietary salt (NaC1) intake on arterial wall function in hypertensive subjects: a randomised controlled cross-over study Investigators: Dr. Rob MacGinley, Professor Robert Walker, Dr. Wayne Sutherland, Alwyn Todd, Professor Jim Mann, Dr. John Schollum Localities: Dept. of Medical and Surgical Sciences Clinic, Endolab, Chch and Royal Perth Hospital APPROVED SUBJECT TO CONDITIONS - Dr. Rob MacGinley, Alwyn Todd and Dr. John Schollum attended the meeting. GENERAL - The Committee notes that the researcher has taken on board previous comments from the Committee BASIC INFO - 4.1 Please confirm that this study is recruiting people with Essential Hypertension only and not normal healthy volunteers - B10 Please confirm that inclusion criteria should read BMI <30 not >30 - D Privacy and Confidentiality – please confirm that potential participants will be made aware by the GP’s of the nature of the personal information that the GP’s are sending to the researchers prior to them consenting to be part of the study and please provide the committee with a copy of the referral form F CULTURAL AND SOCIAL RESPONSIBILITY - F3.1 Please write to Mark Brunton again, in particular with a view to bringing him up to date with the status of the study. Please forward a copy of any response that is received from this communication. DECLARATION FORM - The declaration form needs to be signed by Professor John Highton and a copy forwarded for the Committee’s records INFORMATION SHEET - The committee is aware that some GPs have been approached to facilitate recruiting already and have been provided with an information sheet that is dated June 2006. It is important to note that the study may not begin until full approval has been granted and that potential participants should be provided with the most recent information sheet - Use the preferred ACC statement. See Top ten tips sheet appended. - Pg. 2 Para 3 The second sentence states that the participant should drink 500 mls. of juice for four weeks. Please clarify what is intended. - Include information about parking, taxis and breakfast. - Dr. John Schollum’s name is misspelt in the opening paragraph of the info sheet. Also in the letter to potential participants LOCALITY ASSESSMENT - Please forward copies of the locality assessments as they are received from the University and the ODHB ADVERTISEMENT - Please provide better spacing for the tear of strips. In particular for tearing. Minutes – November Meeting File RE08-04-4 Page – 10 RE-APPROVALS 1. PROJECT KEY: OTA/03/02/009 Full Title: Prospective memory in schizophrenia and related disorders Investigators: Dr Richard Linscott, Associate Professor Robert G Knight, Dr Nick Titov, Mr Mark Lewis Localities: Department of Physiology, University of Otago REAPPROVED 2. PROJECT KEY: LRS/05/17/CPD Full Title: Professional development and recognition programme: portfolio development, is it more than a means to an end? Investigators: Miss Deborah Ashworth Localities: Southland District Health Board REAPPROVED 3. PROJECT KEY: LRS/04/12/005 Full Title: Respiratory and Cardiovascular effects of a bronchodilator drug (200 ug salbutamol) in medicine, pharmacy and pharmacology students. Investigators: Martin J Le Nedelec, Associate Professor Darlington. Localities: School of Medicines. REAPPROVED MATTERS ARISING 1. PROJECT KEY: OTA/02/17/CPD Full Title: Giving asthma and COPD medicines to the residents of Dunedin rest homes Investigators: Gayan Samarakoon, Nerida Smith Localities: Dept of Pharmacology and Toxicology STUDY CLOSED 2. From Theme Action Dr. Khyla Russell Meeting of Māori Members, Ethics Committees, 18 Oct 2006 For your information NOTED CHAIRPERSONS DISCRETIONARY 1. From: Theme: Action: Anthony Molteno The Otago Glaucoma Surgery Outcome Study Ethical approval not required Minutes – November Meeting File RE08-04-4 Page – 11 2. Project Key: LRS/06/08/CPD Full Title: Follow-Up of patients with rheumatoid arthritis Investigators: Deborah Johnstone, Georgina Chan Localities: Department of Rheumatology APPROVED CORRESPONDENCE 1. From: Theme: Action: Health Research Council HRC News Issue No. 53, October 2006 For your information NOTED PROTOCOLS GIVEN FINAL APPROVAL UNDER DELEGATED AUTHORITY 1. PROJECT KEY: LRS/06/09/037 Full Title: The clinical anatomy of the anterior neck muscles Investigators: Ewan Kennedy, Professor Helen Nicholson, Associate Profesor Susan Mercer, Lyndell Kelly Localities: Dunedin Public Hospital, Oncology, Dept. of Anatomy and Structural Biology, Dunedin 2. PROJECT KEY: LRS/06/10/043 Full Title: The geometry of the solid hypophraryngeal walls Investigators: Dr. Ming Zhang, Dr. Amy Fong, Professor Terence Doyle, Dr. Albert Chong Localities: Depts. Anatomy and Structural Biology, Radiology, School of Surveying, University of Otago 3. Project Key: LRS/06/09/038 Full Title: Measurement of quality of life of New Zealand adults and adolescents with cystic fibrosis. Investigators: Malcolm Neall, Dr. Margot Skinner (Supervisor) Localities: Participant’s homes, PI’s home. 4. Project Key: LRS/06/10/045 Full Title: Course and prognosis following first time lateral ankle sprain Investigators: Dr. Joanne Munn, Dr Haxby Abbott Localities: School of Physiotherapy FINAL REPORTS The following final reports have been received. Minutes – November Meeting File RE08-04-4 Page – 12 1. Project Key: LRS/06/05/019 Full Title: A clinical audit of dental implant treatment at the School of Dentistry, 20012005 Investigators: Dr. Warwick Duncan, Ms Angela Ross, Ms Saana Tutbury Localities: University of Otago School of Dentistry - The study is complete however a masters student will be using results from this study for his research. The study was presented at the final year students colloquium day. Smaller samples than originally intended. 117/250 Meeting Ends