POLA_24892_sm_Suppinfo
advertisement

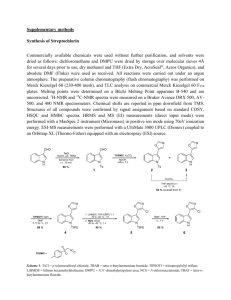
Supporting Information for the paper entitled: Carbohydrate-based crosslinking agents: Potential use in hydrogels STEFAN M. PATERSON,1 JASPER CLARK,1 KEITH A. STUBBS,1 TRAIAN V. CHIRILA,2-5 MURRAY V. BAKER1,2 1 Chemistry M313, School of Biomedical, Biomolecular and Chemical Sciences, The University of Western Australia, Crawley, W.A. 6009, Australia 2 Queensland Eye Institute, 41 Annerley Road, South Brisbane, Queensland 4101, Australia 3 Faculty of Science and Technology, Queensland University of Technology, Brisbane, Queensland 4001, Australia 4 Australian Institute for Bioengineering and Nanotechnology, University of Queensland, St Lucia, Queensland 4072, Australia 5 Faculty of Health Sciences, University of Queensland, Herston, Queensland 4006, Australia Correspondence to: M. V. Baker (E-mail: murray.baker@uwa.edu.au) or K. A. Stubbs (E-mail: keith.stubbs@uwa.edu.au). Contents 1. Materials S2 2. Synthesis of crosslinking agents S3 3. Polymerization S9 4. SEM S9 5. References S10 S1 1. Materials Nuclear magnetic resonance spectra were recorded using Varian 400 (399.9 MHz for 1H and 100.5 MHz for 13C) Bruker 500 (500.1 MHz for 1H and 125 for 13C 150.15 MHz) or Bruker 600 (600.1 MHz for 1H and 150.9 MHz for 13C) spectrometers at ambient temperatures. 1H and 13C spectral assignment were made with the aid of DEPT, HMBC, and HSQC experiments. 1H and 13 C chemical shifts were references to solvent signals. Optical rotation measurements were preformed using a Perkin Elmer 141 polarimeter. High resolution FAB-MS spectra were recorded using VG Auto Mass spectrometer. All solvents were re-distilled prior to use. Anhydrous solvents were obtained by distillation from a suitable drying agent under an inert atmosphere. Flash chromatography was preformed using Merck silica gel 60 (40-63µm). Size-exclusion chromatography was performed using LH-20 Sephadex resin (Aldrich). All reactions were conducted under an inert atmosphere unless stated otherwise. 2-Hydroxyethyl methacrylate (HEMA) (Bimax, Inc, USA > 99.0%) was distilled in batches (b.p. 38-39 °C /0.1 mm Hg) and stored at -20 °C until use. 1,2,3,4,6-Penta-O-acetyl-β-D-glucose (Aldrich, 99%), 2-bromoethanol (Aldrich, 95%), 2,2-dimethoxy-2-phenylacetophenone (DPAP) (Igracure 651, Aldrich, 97 %), tetraethyleneglycol dimethacrylate (TEGDMA) (Fluka), NaN3 (Fluka), BF3.Et2O (Fluka), palladium on carbon (Fluka, puriss 10 % Pd), (1-(3-dimethylaminopropyl)-3-ethyl)carbodiimide hydrochloride (EDC.HCl, Alfa-Aeser), acetic anhydride (Ajax), triphenylmethyl chloride (Sigma), methanesulfonyl chloride (Fluka), sodium methoxide (Sigma) and N-hydroxysuccinimide (Fluka) were all used as received. N-Methacryloyl succinimide (MA-NHS) was prepared according to literature procedures.1 S2 2. Synthesis of crosslinking agent 1 2-Bromoethyl 2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside 3 Boron trifluoride diethyl etherate (7.1 ml, 50 mmol) was added dropwise to a solution of 2bromoethanol (1.6 g, 12 mmol) and 1,2,3,4,6 penta-O-acetyl-β-D-glucose 2 (4.0 g, 10 mmol) in dry CH2Cl2 (30 mL) at 0 °C. The solution was then stirred at room temperature (12 h). The mixture was then concentrated to yield a light brown residue which was subsequently extracted with EtOAc (3 20 mL). The organic fractions were then combined, washed with H2O (20 mL), brine (10 mL) and dried (MgSO4). Concentration, followed by flash chromatography of the residue (EtOAc/hexane, 2:3) yielded 3 as a light brown solid (4.1 g 87%). The 1H and 13C NMR spectra were consistent with those previously reported.2 2-Azidoethyl 2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside 4 Sodium azide (2.9 g, 45 mmol) was added to a solution of 3 (4.1 g, 9.0 mmol) in DMF (100 mL) and stirred (12 h) at 70 °C. The solution was then concentrated to yield a white solid which was subsequently extracted with EtOAc (3 20 mL). The organic fractions were then combined, washed with H2O (20 ml), brine (20 mL) and dried (MgSO4). Concentration followed by followed by flash chromatography of the residue (EtOAc/hexane, 2:3) yielded 4 as a yellow solid (3.4 g, 90%). Rf: 0.57 (SiO2, hexanes/EtOAc, 1:1). Mp: 88-90oC (EtOAc/hexane), [α]D -25.7° (CHCl3). max (KBr)/cm-1 2109 (N3), 1759, 1742 (OCOCH3). 1H NMR (600 MHz, CDCl3) δ 5.21 (dd, J = 9.4, 9.6 Hz 1H), 5.09 (dd, J1 = 9.6 Hz, J2 = 10.0 Hz, 1H), 5.02 (dd, J1 = 7.9 Hz, J2 = 9.4 Hz, 1H), 4.95 (d, J = 7.9 Hz, 1H), 4.24 (dd, J1 = 4.7 Hz, J2 = 12.0 Hz, 1H), 4.15 (dd, J1 = 2.4 Hz, J2 = 12.0 Hz, 1H), 4.02 (ddd, J1 = 1.8 Hz, J2 = 4.0 Hz, J3 = 12.0 Hz, 1H), 3.75-3.65 (m, 2H), 3.49 (ddd, J1 = 3.4 Hz, J2 = 9.0 Hz, J3 = 12.0 Hz, 1H), 3.28 (ddd, J1 = 4.6 Hz, J2 = 5.0 Hz, J3 = 14.0 Hz 1H), 2.08 (s, 3H), 2.04 (s, 3H), 2.02 (s, 3H), S3 1.98 (s, 3H). 13 C HMR (150 MHz, CDCl3) δ 170.6, 170.2, 169.3 (C=O) 100.6 (C1) 72.7, 71.9, 71.4, 68.2, 68.5, 61.8 (C2,3,4,5,6,CH2), 50.5 (CH2N3), 20.7, 20.6, 20.5, 20.5 (CH3). HRMS (m/z): calcd for C16H23N3O10, 417.1337; found, 417.1383 (M)+. 2-Azidoethyl β-D-glucopyranoside 5 Sodium methoxide (20 mg, 0.37 mmol) was added to a solution of 4 (5.9 g, 140 mmol) in methanol (30 mL) and stirred (1 h). The solution was neutralised with resin (Amberlite IR-120[H+]) and then filtered and concentrated yielding the title compound 5 as a colourless oil (3.5 g, 98%). Rf: 0.35 (SiO2, EtOAc/MeOH, 9:1). [α]D -15.7° (MeOH) 1H-NMR (600 MHz, D2O) δ 4.31 (d, J = 7.8 Hz, 1H), 4.02 (ddd, J1 = 3.3 Hz, J2 = 3.3 Hz, J3 = 6.6 Hz, 1H), 3.87 (dd, J1 = 1.6 Hz, J2 = 12.0 Hz, 1H), 3.79-3.70 (m, 3H), 3.67 (dd, J1 = 2.0 Hz, J2 = 4.0 Hz, 1H), 3.50-3.44 (m, 2H), 3.27 (dd, J1 = 2.0 Hz, J2 = 9.0 Hz, 1H), 3.21-3.10 (br s, 4H) 3.18 (dd, J1 = 7.8 Hz, J2 = 9.0 Hz, 1H). 13C-NMR (150 MHz, CDCl3) δ 104.5 (C1) 78.0, 75.1, 71.6, 68.8, 69.3, 61.8 (C2,3,4,5,6,CH2), 49.4 (CH2N3). HRMS (m/z): calcd for C8H15N3O6, 249.0961; found, 249.0963 (M)+. 2-Azidoethyl 6-O-(triphenylmethyl)-β-D-glucopyranoside 6 Triphenylmethyl chloride (4.1 g, 150 mmol) was added to a solution of 5 (3.5 g, 133 mmol), triethylamine (3.3 mL), DMAP (81 mg, 0.70 mmol) in dry CH2Cl2 (30 mL) and the mixture stirred (12 h). The mixture was then quenched (MeOH) and concentrated to yield a yellow solid. This was subsequently extracted with EtOAc (3 30 mL) and the organic fractions were then combined, washed with H2O (20 mL), brine (20 mL) and dried (MgSO4). Concentration followed by followed by flash chromatography of the residue (EtOAc) yielded 6 as a yellow oil (4.7 g, 74%). Rf: 0.70 (SiO2, EtOAc). [α]D - 37.9° (CHCl3). 1H NMR (600 MHz, CDCl3) δ 7.47-7.42 (m, 6H), 7.34-7.28 (m, 6H), 7.28-7.22 (m, 3H), 4.36 (d, J = 7.7 Hz, 1H), 4.07 (ddd, J1 = 3.0 Hz, J2 = 5.4 Hz, J3 = 10.0 Hz, 1H), 3.75 (ddd, J1 = 3.0 Hz, J2 = 7.7 Hz, J3 = 10.0 Hz, 1H), 3.60-3.50 (m, 3H), 3.47-3.34 (m, 5H), 3.15 (s, 3H). 13C HMR S4 (150 MHz, CDCl3) δ 143.5, 128.6, 127.9, 127.1 (Ar), 102.7 (C1), 77.3, 76.1, 74.2, 73.5, 61.9, 68.5, 64.0 (CPh3,C2,3,4,5,6,CH2), 50.7 (CH2N3). HRMS (m/z): calcd for C29H30N3O6, 492.2135; found, 492.2164 (M+H)+. 2-Azidoethyl 2,3,4-tri-O-acetyl-6-O-(triphenylmethyl)-β-D-glucopyranoside 7 Acetic anhydride (2.0 g, 20 mmol) was added to a solution of the alcohol 6 (2.4 g, 4.9 mmol) in pyridine (20 mL) and the mixture stirred (12 h). The mixture was then quenched (MeOH) and concentration of the solution yielded a colourless residue that was subsequently extracted with EtOAc (3 20 mL). The organic fractions were then combined, washed with H2O (30 ml), brine (20 ml) and dried (MgSO4). Concentration followed by flash chromatography of the residue (EtOAc/hexane, 2:3) yielded the title compound 27 as a white solid (2.8 g, 87%). Rf: 0.61 (SiO2, hexanes/EtOAc, 4:1). Mp: 154-156 °C (EtOAc/hexane) [α]D +1.0° (CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.45-7.40 (m, 5H), 7.30-7.18 (m, 10H), 5.15 (dd, J1 = 6.5 Hz, J2 = 6.5 Hz, 1H), 5.15 (dd, J1 = 12.1 Hz, J2 = 18.0 Hz, 1H), 5.06 (dd, J1 = 6.4 Hz, J2 = 7.6 Hz, 1H), 4.58 (d, J = 7.6 Hz, 1H), 4.08 (ddd, J1 = 3.6 Hz, J2 = 4.5 Hz, J3 = 10.0 Hz, 1H), 3.75 (ddd, J1 = 3.1 Hz, J2 = 7.3 Hz, J3 = 10.0 Hz, 1H), 3.58-3.50 (m, 2H), 3.32 (ddd, J1 = 3.1 Hz, J2 = 4.2 Hz, J3 = 12.6 Hz, 1H), 3.25 (dd, J1 = 4.2 Hz, J2 = 18.0 Hz, 1H), 3.09 (dd, J1 = 4.7 Hz, J2 = 18.0 Hz, 1H), 2.04 (s, 3H), 1.97 (s, 3H), 1.71 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 170.6, 169.5, 168.9 (C=O) 143.5, 128.6, 127.7, 127.0 (Ar) 100.6 (C1), 76.6, 73.4, 73.5, 71.2, 68.2, 68.6, 61.8 (CPh3,C2,3,4,5,6,CH2), 50.5 (CH2N3), 20.7, 20.6, 20.3 (CH3). HRMS (m/z): calcd for C33H36N3O9, 617.2373; found, 617.2342 (M)+. 2-Azidoethyl 2,3,4-tri-O-acetyl-β-D-glucopyranoside 8 The triacetate 7 (2.7 g, 4.5 mmol) was dissolved in AcOH:H2O (4:1, 50 mL) and the mixture stirred overnight at 80 °C. The solution was concentrated to yield a yellow residue that was subsequently S5 extracted with EtOAc (3 30 mL). The organic fractions were combined, washed with H2O (30 mL), brine (20 mL) and dried (MgSO4). Concentration followed by flash chromatography of the residue (EtOAc/hexane, 3:7) yielded 8 as a white solid (1.4 g, 81%). Rf: 0.48 (SiO2, hexanes/EtOAc, 3:2). Mp: 91-94°C (EtOAc/hexane) [α]D -45.6° (CHCl3). 1H NMR (400 MHz, CDCl3) δ 5.25 (dd, J1 = 8.8 Hz, J2 = 9.4 Hz, 1H), 5.04 (dd, J1 = 8.8 Hz, J2 = 9.4 Hz, 1H), 4.99 (dd, J1 = 7.6 Hz, J2 = 9.4 Hz, 1H), 4.61 (d, J = 7.6 Hz, 1H), 4.03 (ddd, J1 = 2.9 Hz, J2 = 5.2 Hz, J3 = 10.4 Hz, 1H), 3.75 (dd, J1 = 1.4 Hz, J2 = 11.8 Hz, 1H), 3.70 (ddd, J1 = 2.9 Hz, J2 = 7.4 Hz, J1 = 10.4 Hz, 1H), 3.60 (dd, J1 = 4.4 Hz, J2 = 11.8 Hz, 1H), 3.53 (ddd, J1 = 1.4 Hz, J2 = 4.4 Hz, J3 = 8.8 Hz, 1H), 3.47 (ddd, J1 = 2.9 Hz, J1 = 7.4 Hz, J3 = 11.8 Hz, 1H), 3.30 (ddd, J1 = 2.9 Hz, J2 = 5.2 Hz, J3 = 11.8 Hz, 1H), 2.05 (s, 3H), 2.04 (s, 3H), 2.00 (s, 3H), 2.03 (br s, 1H). 13C NMR (100 MHz, CDCl3) δ 170.2, 170.1, 169.4 (C=O) 100.5 (C1), 74.1, 73.6, 71.2, 68.4, 68.5, 61.3 (C2,3,4,5,6,CH2), 50.5 (CH2N3), 20.7, 20.6, 20.3 (CH3). HRMS (m/z): calcd for C14H21N3O9, 375.1278; found, 375.1253 (M)+. 2-Azidoethyl 2,3,4-tri-O-acetyl-6-O-(methanesulfonyl)-β-D-glucopyranoside 9 Methanesulfonyl chloride (468 µL, 6.05 mmol) was added to a solution of the alcohol 8 (1.4 g, 4.0 mmol) and triethylamine (1.1 mL) in dry CH2Cl2 (20 mL) at -30 °C. The solution was then stirred and allowed to warm to room temperature (2 h). Concentration of the solution yielded a yellow residue that was subsequently extracted with Et2O (3 x 30 mL). The organic fractions were then combined, washed with H2O (20 ml), brine (10 ml) and dried (MgSO4). Concentration followed by flash chromatography of the residue (EtOAc/hexane, 1:3) yielded the 9 as white needles (1.3 g, 72%). Rf: 0.62 (SiO2, hexanes/EtOAc, 3:2). Mp: 83-86°C (EtOAc/hexane). [α]D -21.7° (CHCl3) max (KBr)/cm-1 2109 (N3), 1237 (O=S=O). 1H NMR (400 MHz, CDCl3) δ 5.21 (dd, J1 = 7.5 Hz, J2 = 9.1 Hz, 1H), 5.04 (dd, J1 = 9.1 Hz, J2 = 9.6 Hz, 1H), 5.00 (dd, J1 = 7.5 Hz, J2 = 8.0 Hz, 1H), 4.61 (d, J = 8.0 Hz, 1H), 4.29 (d, J = 2.9 Hz, 2H), 4.02 (ddd, J1 = 2.9 Hz, J2 = 4.4 Hz, J3 = 10.3 Hz, 1H), 3.80 (ddd, J1 = 2.9 Hz, J2 = 2.9 Hz, S6 J3 = 9.6 Hz, 1H), 3.70 (ddd, J1 = 2.9 Hz, J2 = 7.4 Hz, J3 = 10.3 Hz, 1H), 3.47 (ddd, J1 = 2.9 Hz, J2 = 7.4 Hz, J3 = 13.3 Hz, 1H), 3.30 (ddd, J1 = 2.9 Hz, J2 = 4.4 Hz, J3 = 13.3 Hz, 1H), 3.05 (s, 3H), 2.05 (s, 3H), 2.04 (s, 3H), 1.99 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 170.2, 169.5, 169.3 (C=O) 100.5 (C1) 72.4, 71.7, 70.9, 68.1, 68.6, 66.5 (C2,3,4,5,6,CH2), 50.5 (CH2N3), 20.7, 20.6, 20.3 (CH3). HRMS (m/z): calcd for C15H23N3O11S, 453.1053; found, 453.1053 (M)+. Azidoethyl 2,3,4-tri-O-acetyl-6-azido-6-deoxy-β-D-glucopyranoside 10 Sodium azide (950 mg, 14.0 mmol) was added to a solution of the mesylate 9 (1.3 g, 2.90 mmol) in DMF (30 mL) at 0 °C. The solution was then stirred at 70 °C (12 h). Concentration yielded a yellow residue that was subsequently extracted with EtOAc (3 30 mL). The organic fractions were combined, washed with H2O (30 mL), brine (20 mL) and dried (MgSO4). Concentration followed by flash chromatography of the residue (EtOAc/hexane, 1:4) yielded the title compound 10 as a white solid (1.10 g, 92%). Rf: 0.55 (SiO2, hexanes/EtOAc, 3:2). Mp: 79-81oC (EtOAc/hexane). [α]D -65.4o (CHCl3) max (KBr)/cm-1 2148, 2113 (N3). 1H NMR (400 MHz, CDCl3) δ 5.20 (dd, J1 = 9.3 Hz, J2 = 9.7 Hz, 1H), 5.01 (dd, J1 = 8.0 Hz, J2 = 9.3 Hz, 1H), 4.97 (dd, J1 = 9.7 Hz, J2 = 10.2 Hz, 1H), 4.62 (d, J = 8.0 Hz, 1H), 4.05 (ddd, J1 = 2.6 Hz, J2 = 3.4 Hz, J3 = 10.4 Hz, 1H), 3.72-3.66 (m, 2H), 3.48 (ddd, J1 = 2.6 Hz, J2 = 10.4 Hz, J3 = 13.9 Hz, 1H), 3.40 (dd, J1 = 6.9 Hz, J2 = 13.0 Hz, 1H), 3.28 (ddd, J1 = 2.6 Hz, J2 = 3.4 Hz, J3 = 13.9 Hz, 1H), 3.20 (dd, J1 = 1.7 Hz, J2 = 13.0 Hz, 1H), 2.03 (s 3H), 2.02 (s 3H), 1.99 (s 3H). 13 C NMR (100 MHz, CDCl3) δ 170.1, 169.5, 169.3 (C=O) 100.4 (C1), 73.7, 72.4, 71.0, 68.5, 69.5 (C2,3,4,5,CH2), 51.1, 50.4 (C6, CH2N3), 20.7, 20.6, 20.3 (CH3). HRMS (m/z): calcd for C14H20N6O8, 400.1376; found, 400.1343 (M)+. 2-Azidoethyl 6-azido-6-deoxy-β-D-glucopyranoside 11 S7 Sodium methoxide (20 mg, 0.37 mmol) was added to a solution of 10 (1.10 g, 2.70 mmol) in methanol (20 mL) and stirred (1 h). The solution was neutralised with resin (Amberlite IR-120[H+]) and then the solution was then filtered and concentrated yielding the title compound as a white solid (650 mg, 84%). Rf: 0.36 (SiO2, EtOAc). Mp: 71-74°C (MeOH) [α]D -44.3° (MeOH) max (KBr)/cm-1 2109, 2089 (N3). 1 H NMR (400 MHz, CDCl3) δ 3.15 (d, J = 5.7 Hz, 1H), 2.62 (ddd, J1 = 2.5 Hz, J2 = 4.4 Hz, J3 = 10.0 Hz, 1H), 2.44 (ddd, J1 = 2.5 Hz, J2 = 4.4 Hz, J3 = 13.3 Hz, 1H), 2.25- 2.10 (m, 8H), 2.06 (dd, J1 = 4.2 Hz, J2 = 7.1 Hz, 1H), 1.97 (dd, J1 = 5.7 Hz, J2 = 7.1 Hz, 1H), 1.88 (dd, J1 = 4.2 Hz, J2 = 5.7 Hz, 1H). 13 C NMR (100 MHz, CDCl3) δ 100.4 (C1), 75.3, 74.7, 72.9, 68.5, 70.3 (C2,3,4,5,CH2), 50.8, 50.4 (C6, CH2N3). HRMS (m/z): calcd for C8H15N6O5, 275.1104; found, 275.0192 (M+H)+. Anal. calcd for C8H14N6O5: C, 37.74; H, 5.70. Found: C, 37.83; H, 5.75%. 2-Aminoethyl 6-amino-6-deoxy-β-D-glucopyranoside 12 Palladium-on-charcoal (10%, 100 mg) was added to a solution of the diazide 11 (650 mg, 2.2 mmol) in MeOH (20 mL) and the solution stirred under an atmosphere of hydrogen (1 atm., 4 h). The mixture was then filtered through a celite pad and concentrated yielding the title compound as a colourless oil (485 mg, 95%) which was used without further purification. 2-Methacryloylamidoethyl 6-deoxy-6-(2-methacrylamido)-β-D-glucopyranoside 1 To a solution of the diamine 12 (255 mg, 1.1 mmol) in a THF:50 mM NaHCO3 (aq) solution (1:1, 15mL) with triethylamine ( mL) was added a solution of MA-NHS (613 mg, 3.45 mmol) in a THF/H2O solution (1:1, 10 ml). The solution was stirred (12 h) then concentrated and the resultant aqueous solution treated with resin (Amberlite IR-120[H+]) and the resin was eluted with MeOH and then concentrated. Concentration gave a residue which was added to a column of Sephadex (LH-20) and the column eluted with MeOH. Concentration followed by lyophilization gave a white solid. (120 mg, 31%). [α]D -14.7° (H2O). 1H NMR (500 MHz, d6-DMSO) δ 7.80 (br s, 2H), 6.12 (s, 1H), 5.82 (s, 1H), S8 5.65 (s, 1H), 5.32 (s, 1H), 4.12 (d, J = 8.0 Hz, 1H), 3.74 (ddd, J1 = 7.5 Hz, J2 = 8.5 Hz, J3 = 11.0 Hz, 1H) 3.57-3.49 (m, 2H), 3.28-3.19 (m, 2H), 3.17-3.11 (m, 2H), 3.00-2.90 (m, 2H) 2.46-2.42 (m, 2H) 2.39-2.35 (m, 2H), 1.93 (s, 3H) 1.84 (s, 3H) 13 C NMR (125 MHz, d6-DMSO) δ 167.9, 167.6 (C=O), 139.8, 139.8 (C=CH2), 119.2, 119.1 (C=CH2), 103.2 (C1), 76.0, 74.2, 73.5, 72.0, 67.5 (C2,3,4,5,CH2), 41.1 39.8 (C6, CH2NH), 18.6, 18.5 (CH3). HRMS (m/z): calcd for C16H27N2O7, 359.1818; found, 359.1803 (M+H)+. Anal. calcd for C16H26N2O8: C, 53.62; H, 7.31. Found: C, 53.58; H, 7.40% 3. Polymerization studies Polymer hydrogels were prepared as described previously3 by photoinitiated polymerization of HEMA. Briefly, DPAP (0.1 mol% with respect to HEMA) was added to a 80:20 H2O:HEMA solution containing 1 or TEGDMA (1 mol% with respect to HEMA) and the solution was irradiated under a UV lamp (UVP Blak-Ray®, 365 nm, 120 W) for 30 min. After polymerization, the hydrogels were soaked in water for one week to remove any unreacted monomers, with water being exchanged daily. After soaking, all polymer samples were cut into 300-µm thick cross-sections (Vibratome 3000) and these sections were further cut into disks using a 5-mm biopsy cutter. After sectioning, the samples were carefully transferred with soft plastic tweezers into vials of deionized water where they were stored until required. 4. SEM Samples were dehydrated by freeze-drying (Dynavac FD2) and then were mounted on double-sided carbon tabs and coated with a layer of carbon (approximately 30 nm thick) using a carbon evaporator (Speedivac 12E6/1178, Edwards High Vacuum LTD). The samples were then imaged by SEM (Zeiss 1555 VF-FESEM) at 3 kV, using a working distance of 6 mm and an aperture of 10 µm. To acquire an image, frame integration was used to prevent charging on the surface of the polymer. S9 References 1. Monge, S, Haddleton, DM. Eur Polym J 2004, 40, 37–45 2. Dahmén J, Frejd T, Grönberg G, Lave T, Magnusson G, Noori G. Carbohydr Res 1983, 116, 303-307. 3. Baker MV, Brown DH, Casadio YS, Chirila TV. Polymer 2009, 50, 5918-5927. S10