BUVANEST - Perdossi
advertisement

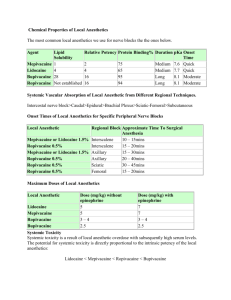
English Version BUVANEST® Description Buvanest® is a sterile, isobaric, isotonic solution that contains bupivacaine HCl for local anaesthesia and administered parenterally by injection. Buvanest® Spinal 0,5 % Heavy is a sterile, hiperbaric, isotonic solution that contains bupivacaine HCl and dextrose monohydrate for local anaesthesia and administered parenterally by injection. Composition Each ampoule of Buvanest® 0.5 % contains : Bupivacaine HCl 5 mg/mL Each ampoule of Buvanest® Spinal 0.5 % Heavy contains : Bupivacaine HCl 5 mg/mL and Dextrose monohydrate 80 mg/mL Pharmacology Pharmacodynamics Local anaesthetics block the generation and the conduction of nerve impulses, by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anaesthesia is related to the diameter, myelination and conduction velocity of affected nerve fibers. At blood concentrations achieved with therapeutic doses, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance are minimal. Toxic blood concentrations may lead to atrioventricular block, ventricular arrhythmias and to cardiac arrest, sometimes resulting in fatalities. Myocardial contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure. Systemic absorption of local anaesthetics can also produce central nervous system stimulation, depression or both. Central stimulation is usually manifested as restlessness, tremors and shivering, convulsions, followed by depression and coma, progressing ultimately to respiratory arrest. The local anesthetics have a primary depressant effect on the medulla and on higher centers. The depressed stage may occur without a prior excited stage. Pharmacokinetics The rate of systemic absorption of local anaesthetics is dependent upon the total dose and concentration of drug administered, the route of administration, the vascularity of the administration site, and the presence or absence of epinephrine in the anaesthetic solution. After injection of bupivacaine for caudal, epidural or peripheral nerve block in man, peak levels of bupivacaine in the blood are reached in 30 to 45 minutes, followed by a decline to insignificant levels during the next 3 to 6 hours. Depending upon the route of administration, local anaesthetics are distributed to some extent to all body tissues, with high concentrations found in highly perfused organs such as the liver, lungs, heart, and brain. Bupivacaine is metabolized primarily in the liver via conjugation with glucuronic acid and the major metabolite is 2,6-pipecoloxylidine. The onset of action with bupivacaine is rapid and anaesthesia is long-lasting. The duration of anaesthesia is significantly longer with bupivacaine than with any other commonly used local anaesthetic. Also there is a period of analgesia that persists after the return of sensation. Local anaesthetics are bound to plasma proteins in varying degrees. The half-life of bupivacaine in adults is 2.7 hours and in neonates 8.1 hours. The duration of analgesia from bupivacaine 0,5 % is between 3-5 hours in the lower thoracic and lumbar segments, and that from the bupivacaine 0,5 % with dextrose is between 2-3 hours in the T10-T12. the bupivacaine 0,5 % produces muscle relaxation in the lower limbs which lasts 3-4 hours, and the bupivacaine 0,5 % with dextrose is 2-3 hours. Motor blockade of the abdominal muscles makes bupivacaine 0,5 % with dextrose suitable for performance of abdominal surgery lasting 45-60 minutes. The kidney is the main excretory organ for most local anaesthetics and their metabolites. Urinary excretion is affected by renal perfusion and factors affecting urinary pH. Only 5% of bupivacaine is excreted unchanged in the urine. When administered in recommended doses and concentrations, bupivacaine does not ordinarily produce irritation or tissue damage and does not cause methemoglobinemia. Various pharmacokinetic parameters of the local anaesthetics can be significantly altered by the presence of hepatic or renal disease, addition of epinephrine, factors affecting urinary pH, renal blood flow, the route of drug administration, and the age of the patient. Elderly patients reached the maximal spread of analgesia and maximal motor blockade more rapidly than younger patients. Elderly patients also exhibited higher peak plasma concentrations following administration of this product but total plasma clearance was decreased in these patients. Indications Buvanest® 0,5 % is indicated for local or regional anaesthesia or analgesia for surgery, for oral surgery procedures, for diagnostic and therapeutic procedures, and for obstetrical procedures. Buvenest® Spinal 0,5 % Heavy is indicated for spinal anaesthesia for abdominal , urological, and lower limb surgery. Contraindications Buvanest® is contraindicated for : - Obstetrical paracervical block anaesthesia. - Intravenous regional anaesthesia. - Patients with a known hypersensitivity to bupivacaine or to other local anaesthetic agents of the amide type or to other components of Buvanest® solutions. Warnings and Precautions - Local anaesthetics should only be employed by clinicians who well versed in diagnosis and management of dose-related toxicity and other acute emergencies, and after insuring the immediate availability of oxygent, other resuscitative drugs, cardiopulmonary resuscitative equipment, and the personnel resources needed for proper management of toxic reactions and related emergencies. - During epidural administration, Buvanest® should be administered in incremental doses of 3 mL to 5 mL with sufficient time between doses administration. Administration of Buvanest® is not recommended for children younger than 12 years. Patients over 65 years, should be taken in dose selection, and it may be useful to monitor renal function. Should be used cautiously in patients with renal, hepatic, and cardiovascular function impairment. Mixing bupivacaine with other local anesthetics cannot be recommended. It is essential that aspiration for blood or cerebrospinal fluid be done prior to injecting any local anaesthetic to avoid intravascular or subarachnoid injection. Buvanest should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Bupivacaine is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from bupivacaine, a decision to discontinue nursing or not administer bupivacaine depending on the importance of the drug to the mother. Adverse Effects A major cause of adverse reactions to this group of drugs may be associated with its excessive plasma levels, which may be due to overdosage, unintentional intravascular injection or slow metabolic degradation. Systemic : The commonly are related to the central nervous system and the cardiovascular system such as underventilation or apnea, hypotension, and cardiac arrest. CNS : Restlessness, anxiety, dizziness, tinnitus, blurred vision or tremors may occur, possibly proceeding to convulsions. This may quickly be followed by drowsiness merging into unconsciousness and respiratory arrest. Other central nervous system effects may be nausea, vomiting, chills, and constriction of the pupils. Cardiovascular : Depression of the myocardium, decreased cardiac output, heart block, hypotension, bradycardia, ventricular arrhythmias, including ventricular tachycardia and ventricular fibrillation, and cardiac arrest. Allergic : Urticaria, pruritus, erythema, angioneurotic edema (including laryngeal edema), sneezing, asthmatic episodes and possibly, anaphylactoid symptomatology (including severe hypotension). Neurologic : Paralysis of the legs, loss of consciousness, respiratory paralysis and bradycardia (high spinal), hypotension secondary to spinal block; urinary retention; fecal and urinary incontinence; loss of perineal sensation and sexual function; persistent anesthesia, paresthesia, weakness, paralysis of the lower extremities and loss of sphincter control, headache; backache; septic meningitis; meningismus; slowing of labor; increased incidence of forceps delivery; or cranial nerve palsies due to traction on nerves from loss of cerebrospinal fluid. Dose and Administration Buvanest® 0,5 % in adult patients : - Peripheral nerve block Epidural block Caudal block : 5 mL to maximal : 10-20 mL : 15-30 mL Buvanest® Spinal 0,5 % Heavy in adult patients : The dose should be regarded as a guide for average use in adults. Spinal anaesthesia for surgery : 1.5-3 mL/7.5-15 mg bupivacaine HCl. When injected 1.5 – 3 mL of bupivacaine HCl in the L3/4/5 interspace with the patient in the sitting position, the blockade spreads to T7-T10. Of the patient is in the supine horizontal position, the blockade spreads to T4-T7. The effects of injection of bupivacaine HCl exceeding 4 mL have not yet been studied and such volumes can there for not be recommended. The solution should be used immediately after opening the ampoule. Any remaining solution should be discarded. Presentation Buvanest® 0,5 % Buvanest® Spinal 0,5 % Heavy Ampoule 20 mL Ampoule 4 mL Reg.No. Reg.No. Store Buvanest® preparations at room temperature (15-30°C ). Should not be used if its color is pinkish or darker than slightly yellow or if it contains a precipitate. Should be used immediately after opening the ampoule, any remaining solution should be discarded.