Controls on the Chemical Composition of Crustal Brines
advertisement
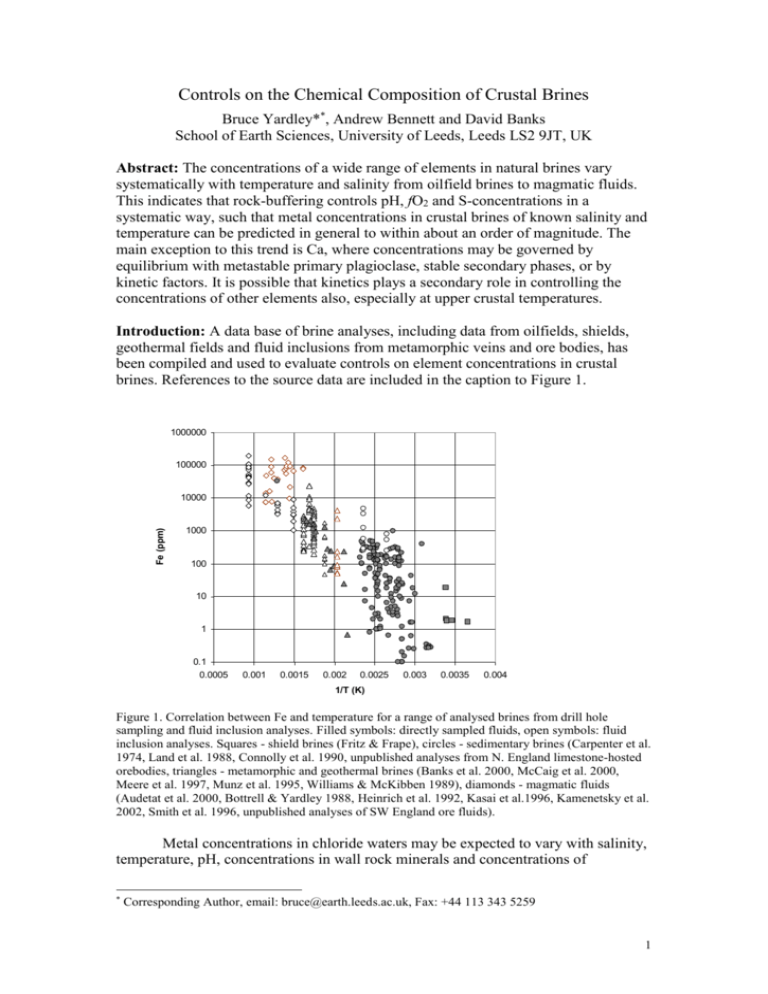
Controls on the Chemical Composition of Crustal Brines Bruce Yardley**, Andrew Bennett and David Banks School of Earth Sciences, University of Leeds, Leeds LS2 9JT, UK Abstract: The concentrations of a wide range of elements in natural brines vary systematically with temperature and salinity from oilfield brines to magmatic fluids. This indicates that rock-buffering controls pH, fO2 and S-concentrations in a systematic way, such that metal concentrations in crustal brines of known salinity and temperature can be predicted in general to within about an order of magnitude. The main exception to this trend is Ca, where concentrations may be governed by equilibrium with metastable primary plagioclase, stable secondary phases, or by kinetic factors. It is possible that kinetics plays a secondary role in controlling the concentrations of other elements also, especially at upper crustal temperatures. Introduction: A data base of brine analyses, including data from oilfields, shields, geothermal fields and fluid inclusions from metamorphic veins and ore bodies, has been compiled and used to evaluate controls on element concentrations in crustal brines. References to the source data are included in the caption to Figure 1. 1000000 100000 Fe (ppm) 10000 1000 100 10 1 0.1 0.0005 0.001 0.0015 0.002 0.0025 0.003 0.0035 0.004 1/T (K) Figure 1. Correlation between Fe and temperature for a range of analysed brines from drill hole sampling and fluid inclusion analyses. Filled symbols: directly sampled fluids, open symbols: fluid inclusion analyses. Squares - shield brines (Fritz & Frape), circles - sedimentary brines (Carpenter et al. 1974, Land et al. 1988, Connolly et al. 1990, unpublished analyses from N. England limestone-hosted orebodies, triangles - metamorphic and geothermal brines (Banks et al. 2000, McCaig et al. 2000, Meere et al. 1997, Munz et al. 1995, Williams & McKibben 1989), diamonds - magmatic fluids (Audetat et al. 2000, Bottrell & Yardley 1988, Heinrich et al. 1992, Kasai et al.1996, Kamenetsky et al. 2002, Smith et al. 1996, unpublished analyses of SW England ore fluids). Metal concentrations in chloride waters may be expected to vary with salinity, temperature, pH, concentrations in wall rock minerals and concentrations of * Corresponding Author, email: bruce@earth.leeds.ac.uk, Fax: +44 113 343 5259 1 precipitating ligands, such as H2So. Despite the wide range of host rocks from which the brines were sourced, the effect of rock-buffering on pH and aH2So is evidently rather consistent, so that chloride concentration and temperature emerge as the major controls on the concentrations of most metals in crustal brines. Na/K ratios vary systematically with temperature for fluids including oilfield brines and metamorphic and magmatic fluids. The principal exceptions are very low temperature brines (<100oC), where anomalously high K levels may reflect the metastable replacement of K-feldspar by kaolinite. There is a strong correlation between concentration and temperature for all transition metals for which reasonable data is available, i.e. Fe, Mn, Pb and Zn. There is insufficient data for Cu to demonstrate clear trends, but it appears to display less of a temeprature dependence than the other metals. Figure 1 demonstrates a 6 orders of magnitude variation in mean Fe in brines between c. 50o and 750oC, with c. 3 orders variation possible at any one T. Fe is inferred to occur in solution as divalent Fe-chloride species at all temperatures, because the mol ratio (Fe/Cl2) remains approximately constant over large ranges of salinity, with a value that increases systematically with temperature (Figure 2). Part of the variability in Fe concentrations is a response to fluctuations in redox, as monitored by Mn/Fe ratios, however analytical and sampling errors may also play a significant part. It is notable that the temperature dependence of Fe-levels appears to be more important relative to wall rock controls on pH, than was indicated by the interpretation of their experimental results on Fe-chloride complexing presented by Heinrich & Seward (1990). 0 -1 2 log mol (Fe/Cl ) -2 -3 Shield Brines (Fritz & Frape 1987) -4 Mole Granite (Audetat et al., 2000) Pyrenees (McCaig et al. 2000) -5 Central Mississippi brines (Carpenter et al. 1974) Offshore Louisiana brines (Land et al. 1988) Capitan Pluton (New Mexico) (Campbell et al 1995) -6 Cornwall (Bottrell& Yardley 1988, Smith et al 1996) Kakkonda Granite Brine (Japan) (Kasai et al 1996) -7 0 100000 200000 Cl (ppm) 300000 400000 Alberta Basin Brines (Connolly et al. 1990) 500000 Industrialnoe T in Deposit ( Kamenetsky et al. 2002) Figure 2. Plot of the mol ratio of Fe to Cl2 as a function of chlorinity for a series of individual data sets from the compilation. Note that the value of this ratio increases to higher temperatures, but for individual data sets, each representing a narrow temperature range, there is little systematic variation between moderately saline and hypersaline fluids.. Zinc solubility has a similar temperature dependence to Fe, with absolute concentrations about 1 order lower than Fe at all temperatures. Individual data sets of fluids formed at the same temperature, ranging from oilfield brines to magmatic fluids, have a slope close to 2 on a plot of log Cl vs log Zn, suggesting that ZnCl2o 2 complexes dominate in nature, even at very high salinities and at magmatic temperatures. Despite the generally good correlation of Zn with T, some longer residence time brines do appear to display relatively high Zn levels, suggesting that kinetic controls on Zn concentrations are not insignificant. Pb varies rather less than Zn with temperature overall, but in detail the Zn/Pb ratio varies systematically (Figure 3) for fluids above about 120oC. Oilfield brines from Mississippi have Zn/Pb ratios of around 6, i.e. close to a continental crust average, but the most metal-enriched magmatic fluids in the compilation show relative enrichment in Pb, with Zn/Pb < 2. 1000000 100000 Zn (ppm) 10000 1000 100 10 1 0.1 1 10 100 1000 10000 Pb (ppm) Figure 3. Correlation between Zn and Pb for crustal brines ranging from low-T shield brines (lower left) to magmatic fluids (upper right), symbols as in Figure 1. Note that the well-defined trend from sedimnatary fluids (Carpenter et al., 1974) to magmatic fluids does not correspond to a constant Zn/Pb ratio. The systematic temperature-dependence of the concentrations of chalcophile elements in natural brines can only arise if the geochemical environment in the crust is strongly buffered by mineral assemblages within narrow limits, and if metal concentrations are controlled by mineral equilibria. The window of possible pH values for a given fluid salinity is rather narrow for the majority of rock buffers, and redox must also vary within quite narrow limits. Pyrite then becomes the control on Slevels for most rock types, and this in turn limits concentrations of elements such as Pb or Zn. In contrast to the transition metals, Ca in natural brines varies greatly and does not appear to be controlled by plagioclase equilibria except at the highest temperatures. Very high Ca/Na ratios can occur in brines from a wide range of temperatures, and may be related to residence time. Within some individual data sets there is a correlation between Ca/Na and Cl, as would be predicted for mineral buffering, but the mineralogical controls may be secondary Ca-Al silicates and albite rather than the primary plagioclase that is decomposing in the same rocks. Thus kinetic controls play a major role in determining the Ca-content of natural brines, and 3 this will in turn limit the levels to which potential complexing ligands such as F may be present in the fluids. References Audetat, A., Gunter, D. & Heinrich, C.A. 2000. Causes for large-scale metal zonation around mineralised plutons: Fluid inclusion LA-ICP-MS evidence from the Mole granite, Australia. Economic Geology 95, 1563-1581. Banks, D.A., Guiliani, G. Yardley, B.W.D. & Cheillitz, A. 2000. Emerald mineralisation in Columbia: Fluid chemistry and the role of brine mixing. Mineralium Deposita 35, 699-713. Bottrell, S.H. & Yardley, B.W.D. 1988. The composition of a primary granite-derived ore fluid from SW England, determined by fluid inclusion analysis. Geochimica et Cosmochimica Acta 52, 585-588. Campbell, A.R., Banks, D.A., Phillips, R.S. & Yardley, B.W.D. 1995. Geochemistry of Th-U-REE mineralizing magmatic fluids, Capitan Mountains, New Mexico. Economic Geology 90, 1271-1287. Carpenter, A.B., Trout, M.L. & Pickett, E.E. 1974. Preliminary report on the origin and chemical evolution of lead and zinc-rich oilfield brines in Central Mississippi. Economic Geology 69, 1191-1206. Connolly, C.A., Walter, L.M., Baadsgaard, H. & Longstaffe, F. 1990. Origina nad evolution of formation waters, Alberta Basin, Western Canada Sedimentary Basin. I. Chemistry. Applied Geochemistry 5, 375-395. Fritz, P. & Frape, S.K. (Eds.) 1987. Saline water and gases in crystalline rocks. Geological Association of Canada Special Paper 33. Heinrich, C.A., Ryan, C.G., Mernagh, T.P. & Eadington, P.J. 1992. Segregation of ore metals between magmatic brine and vapor: a fluid inclusion study using PIXE microanalysis. Economic Geology 87, 1566-1583. Heinrich, C.A. & Seward, T.M. 1990. A spectrophotometric study of aqueous iron (II) chloride complexation from 25 to 200oC. Geochimica et Cosmochimica Acta 54, 2207-2221. Kasai, K., Sakagawa, Y., Miyazaki, S., Sasaki, M. & Uchida, T. 1996. Supersaline brine obtained from Quaternary Kakkonda granite by the Nedo’s deep geothermal well WD-1A in the Kakkonda geothermal field, Japan. Geothermal Resources Council Transactions 20, 623-629. Kamenetsky, V.S., van Achterbergh, E., Ryan, C.G., Naumov, V.B., Menagh, T.P. & Davidson, P. 2002. Extreme chemical heterogeneity of granite-derived hydrothermal fluids: an example from inclusions in a single crystal of miarolitic quartz. Geology 30, 459-462. Land, L.S., McPherson, G.L. & Mack, L.E. 1988. The geochemistry of saline formation waters, Miocene offshore Louisiana. Gulf Coast Association of Geologists Transactions 38, 503-511. McCaig, A.M., Tritlla, J. & Banks, D.A. 2000. Fluid mixing and recycling during Pyrenean thrusting: evidence from Fluid inclusion halogen ratios. Geochimica et Cosmochimica Acta 64, 3395-3412. Meere, P.A. & Banks, D.A. 1997. Upper crustal fluid migration: an example from the Variscides of SW Ireland. Journal of the Geological Society, London 154, 975-985. Munz, I.A., Yardley, B.W.D., Banks, D.A. & Wayne, D. 1995. Deep penetration of sedimentary fluids in basement rocks from southern Norway: evidence from 4 hydrocarbon and brine inclusions in quartz veins. Geochimica et Cosmochimica Acta 59, 239-254. Smith, M.P., Banks, D.A., Yardley, B.W.D. & Boyce, A. 1996. Fluid inclusion and stable isotope constraints on the genesis of the Cligga Head Sn-W deposit, SW England. European Journal of Mineralogy 8, 961-974. Warren, E.A. & Smalley, P.C. (Eds) 1994. Atlas of North Sea Formation Waters. Geological Society of London Memoir 15, 208pp. Williams, A.E. & McKibben, M.A. 1989. A brine interface in the Salton Sea geothermal system, California: fluid geochemical and isotopic characteristics. Geochimica et Cosmochimica Acta 53, 1905-1920. 5