Chromosome conformation capture[1], or 3C, is a high
advertisement
![Chromosome conformation capture[1], or 3C, is a high](http://s3.studylib.net/store/data/007705586_2-3920b3207180094524328ceb0558e139-768x994.png)
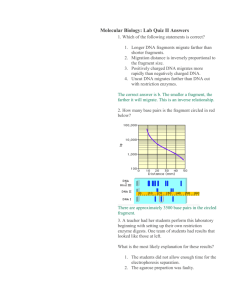
1 Chromosome conformation capture[1], or 3C, is a high-throughput molecular biology technique used to analyze the organization of chromosomes in a cell's natural state. Studying the structural properties and spatial organization of chromosomes is important for the understanding and evaluation of the regulation of gene expression, DNA replication and repair, and recombination. One example of chromosomal interactions influencing gene expression is a chromosomal region which can fold in order to bring an enhancer and associated transcription factors within close proximity of a gene, as was first shown in the beta-globin locus[2]. Chromosome conformation capture has enabled researchers to study the influences of chromosomal activity on the aforementioned cellular mechanisms. This technology has aided the genetic and epigenetic study of chromosomes both in model organisms and in humans. Several techniques have been developed from 3C to increase the throughput of quantifying a chromosome’s interactions with other chromosomes and with proteins. Examples of these technologies are pC, dC, and ChIP-loop. The application of analyzing DNA segments by microarray and high-throughput sequencing in the 4C and 5C methodologies has brought the assessment of chromosome interactions to the genome-wide scale. Contents * 1 Chromosome Conformation Capture (3C) * 2 Circularized Chromosome Conformation Capture (4C) * 3 Carbon-Copy Chromosome Conformation Capture (5C) * 4 ChIP-loop * 5 Advantages and Disadvantages * 6 History * 7 References * 8 See also [edit] Chromosome Conformation Capture (3C) The basic 3C technique has five experimental steps: Step 1 Cross-linking: Addition of formaldehyde results in the cross-linking of DNA segments to proteins and to cross-linking of proteins with each other this leads to cross-linking of interacting DNA segments (for example cis located promoters to trans located promoters, reveals interaction like the one of interaction between H enhancer and odorant receptor promoters). Step 2 Restriction digest: A restriction enzyme is added in excess to the cross-linked DNA, separating the non-cross-linked DNA from the cross-linked chromatin. The selection of the restriction enzyme in this step depends on the locus being analyzed and allows the separate analysis of different regulatory elements. Frequently cutting enzymes (4 bp) are used in studying small loci (<10–20 kb), while larger loci demand the use of larger cutters (6 bp). Restriction enzyme selection also has an impact on digestion efficiency of cross-linked DNA. Step 3 Intramolecular Ligation: Over-represented Ligation Junctions.jpg Using very low concentrations of DNA favors the ligation of relevant DNA fragments with the corresponding junctions instead of the ligation of random fragments. There are two major types of ligation junctions that are over-represented. One is the junction that forms between neighboring 2 DNA fragments due to incomplete digestion, which represents about 20-30% of all junctions. This number can be decreased by reducing the cross-linking stringency in the first step. The other type of junctions over-represented here is the junction that forms when one end of the fragment ligates with the other end of the same fragment, and contributes up to 30% of all junctions formed[3]. Step 4 Reverse Cross-links: High temperature will result in the reversal of cross-links formed in step 1. The resulting linear DNA fragment has specific restriction ends as well as a central restriction site corresponding to the site of ligation. The pool of these fragments is collectively referred to as the 3C library. Step 5 Quantitation: Polymerase chain reaction (PCR) uses primers against the site of ligation to semi-quantitatively assess the frequencies of a restriction fragment of interest. Real-time PCR using Taqman probes (3C-qPCR) provides a more quantitative measurement of the fragment of interest. The Taqman probe and a constant primer hybridize to the restriction fragment that contains the site of contact and one test primer is designed against each neighboring restriction fragments. Together the probe and primers allow for a specific fluorescent signal to be emitted during amplification[4]. [edit] Circularized Chromosome Conformation Capture (4C) The 4C strategy[5][6] has a significant advantage over 3C in that only the sequence of one of the site of interest needs to be known. The fragment, known as the “bait”, contains the site that associates with other chromosomal regions. The 4C procedure follows the same steps as in 3C, except additional processing is needed before quantification of the fragments of interest. Steps 1-4: See procedure listed in 3C. 6-bp-cutters are preferred in step 2. Step 5a Second Restriction Digest: After the reversal of the cross-linked DNA, the restriction fragments are subjected to another round of restriction digest, this time with a frequent cutter that will result in smaller fragments with restriction ends that differ from the central restriction site (ligation junction). Step 5b Self-circularization: Self-circularization of the DNA fragments is more favored now that they are not bound to other proteins or fragments. Intramolecular ligation occurs to induce the formation of the circular fragments. The pool of circular fragments becomes the 4C library. Step 5c Inverse PCR and Quantitation: Primers are designed against the outer restriction sites of the “bait” sequence, which result in the amplification of the small unknown captured fragment. Largescale sequencing can be used to sequence the 4C library. Custom microarrays can also be made using probes designed against the adjacent upstream and downstream regions of all genomic sites of the restriction enzyme used in step 2. [edit] Carbon-Copy Chromosome Conformation Capture (5C) The 5C technique[7] expands from 3C and allows for the parallel analysis of interactions between many selected loci. Steps 1-4: Same as in 3C. Step 5 Ligation-mediated amplification and Quantitation: Performing multiplex ligation-mediated amplification (LMA) after the construction of the 3C library leads requires using multiplex primers that consist of universal primer sequences like T7 and T3 and the ligation junction sequences. They anneal to the 3C fragments and get ligated together with a DNA ligase. Perfect alignment with the 3C template ensures the success of the ligation. The ligated primers serve as templates of which get 3 amplified to generate the 5C library. The use of universal primer sequences mean these 5C fragments can be analyzed on microarrays. The small size (~100 bp) of the 5C fragments is also compatible for analysis using high-throughput sequencing. [edit] ChIP-loop This method[8] is slightly different from the previous techniques in that the interaction formed between two chromosomal regions is mediated by a bound protein. Like in the 5C methodology, a single DNA site is often considered to interact with multiple other sites. After the cross-linking and digestion, ChIP is performed to pull down the protein bound to the site of interest. Normal 3C procedures are conducted after this step. [edit] Advantages and Disadvantages A significant confounding factor in the 3C technology is the frequent random collisions of chromosomal regions to one another, which means that the detection of a product does not always mean a specific interaction has occurred between two regions. Therefore, a specific interaction between two regions is only confirmed when the interaction occurs at a higher frequency than with neighboring DNA. The ChIP-loop may be useful in identifying long-range cis-interactions and trans interaction mediated through proteins since frequent DNA collisions will not occur. The 5C technique overcomes the junctional problems at the intramolecular ligation step and is useful for construct complex interactions of specific loci of interest. This approach is unsuitable for conducting genome-wide complex interactions since that will require millions of 5C primers to be used. In contrast to 3C and 5C, the 4C technique does not require the prior knowledge of both interacting chromosomal regions. Results obtained using 4C are highly reproducible with most of the interactions that are detected are between regions proximal to one another. On a single microarray, approximately a million interactions can be analyzed. A common problem in all of these techniques is the requirement of a great number of cells, especially in the high-throughput methodologies. A single mammalian cell only provides two copies of any given restriction fragment, which can be ligated to only one other partner in 3C. Therefore any kind of quantitative analysis requires a large number of cells due to the need to determine the specification of an interaction between two regions. Experiments using the 4C technique routinely process ten million cells for analysis on a single microarray. [edit] History The 3C methodology was originally developed by Dekker and colleagues in 2002 at the University of Massachusetts and it aimed at identifying, locating and mapping physical interactions between genetic elements located throughout the human genome. This technology would give beneficial insights into the complex interplay of genetic factors that contribute to such debilitating disorders such as cancer, Duchenne muscular dystrophy (DMD), Rett syndrome and Alzheimer's disease. 3C is based on proximity ligation, which has been used extensively in the past decades for other application such as in experiments that aim to determine circularization frequencies of DNA in solution, and the effect of protein-mediated DNA bending on circularization. Other important early studies used proximity ligation in nuclei: These studies were done by Seyfred group (Cullen et a. Science. 1993 Jul 9;261(5118):203-6);(Gothard LQ et al. Mol Endocrinol. 1996 Feb;10(2):185-95). 4 Only the difference was restriction enzyme digestion of unfixed nuclei and ligation insitu without diluting the chromatin. They referred this procedure as "Nuclear Ligation Assay". [edit] References 1. ^ Dekker J, Rippe K, Dekker M, Kleckner N (2002). "Capturing chromosome conformation". Science 295 (5558): 1306–1311. doi:10.1126/science.1067799. PMID 11847345. 2. ^ Tolhuis B, Palstra RJ, Splinter E, Grosveld F and de Laat W (2002). "Looping and interaction between hypersensitive sites in the active beta-globin locus". Mol. Cell. 10 (6): 1453–1465. PMID 12504019. 3. ^ Dekker J. Personal communication. 4. ^ Hagège H, Klous P, Braem C, Splinter E, Dekker J, Cathala G, de Laat W, Forné T (2007). "Quantitative analysis of chromosome conformation capture assays (3C-qPCR)". Nat. Protoc. 2 (7): 1722–1733. doi:10.1038/nprot.2007.243. PMID 17641637. 5. ^ Zhao Z, Tavoosidana G, Sjölinder M, Göndör A, Mariano P, Wang S, Kanduri C, Lezcano M, Sandhu KS, Singh U, Pant V, Tiwari V, Kurukuti S, Ohlsson R (2006). "Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions". Nat. Genet. 38 (11): 1341-1347. doi::10.1038/ng1891. PMID 17033624. 6. ^ Simonis M, Klous P, Splinter E, Moshkin Y, Willemsen R, de Wit E, van Steensel B, de Laat W (2006). "Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C)". Nat. Genet. 38 (11): 1348–1354. doi:10.1038/ng1896. PMID 17033623. 7. ^ Dostie J, Dekker J (2007). "Mapping networks of physical interactions between genomic elements using 5C technology". Nat. Protoc. 2 (4): 988–1002. doi:10.1038/nprot.2007.116. PMID 17446898. 8. ^ Simonis M, Kooren J, and de Laat W (2007). "An evaluation of 3C-based methods to capture DNA interactions". Nat. Methods. 4 (11): 895–901. doi:10.1038/nmeth1114. PMID 17971780. * http://www.nature.com/ejhg/journal/v13/n9/full/5201464a.html * http://www.abcam.com/index.html?pageconfig=resource&rid=10010&pid=5