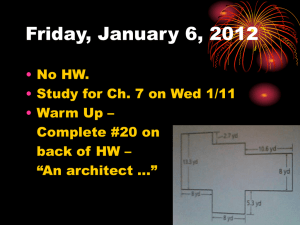
Density review
advertisement

Part 4 Fluidics 121 Density review W Weight W Weight density, D , Volume V D V is the weight in pounds (grams) per unit volume in ft3 or gallons (cm3). Density has units of lb/ft3 or lb/gal or g/cm3. The greater the density, the more matter per volume Example: Answer: Example: balloon? A block has a weight of 16.4 lbs and a volume of 6 ft3. What is the density of the block? D W 16.4lb lb 2.73 3 3 V 6 ft ft A helium filled balloon has a volume of 0.33 ft3. What is the weight of the helium in the Answer: Using the circle above, solve for the weight. You will also need the density of helium, from the table on the next page, DHE=0.011 lbs/ft3. W DV 0.011 lb 0.33 ft 3 0.0036lb ft 3 Example: grams? An iron block measure 22 cm by 57 cm by 9 cm. How mcuh does the block wiegh in Answer: First we need to find the volume of the block. Volume l w h 22cm57cm9cm 11,286cm3 Use the circle above and solve for the weight. You will also need the density of iron in the table on the next page. W DV 7.80 g 11286cm 3 88031g 3 cm Example: What is the volume of 2000 lb of oil? Answer: Solve the circle above for volume and use the density of oils from the table; V W 2000lb 36.9 ft 3 lb D 54.2 3 ft 122 Densities of Common Substances Substance Weight Density (lb/ft3) Mass Density (g/cm3) Solids Aluminum 169 2.7 Brass 527 8.44 Concrete 145 2.3 Copper 555 8.89 Cork 15 0.240 Gold 1213 19.4 Ice 57 0.917 Iron 490 7.80 Lead 708 Wood, pine Zinc 11.3 26 0.420 446 7.133 Liquids Alcohol 49.4 0.79 Gasoline 42.0 0.680 Mercury 846 13.6 Oil 54.2 0.870 Sea water 64.0 1.025 Water 62.4 2 Gases – At 0 °C (32 °F) and a pressure of 14.7 lb/in ( 1 atm) 1.000 Air 0.081 0.00129 Ammonia 0.047 0.00076 Carbon dioxide 0.123 0.00196 Carbon monoxide 0.078 0.00125 Helium 0.011 0.000178 Hydrogen 0.0056 0.0000899 Nitrogen 0.078 0.00125 Oxygen 0.089 0.00143 Propane 0.126 0.00202 123 Pressure F P F Pressure is the force per area P A A or Example: The pressure of a phonograph needle on a record. Weight of needle = 0.02 lbs. The area of contact = 0.0001 in2. P Answer: F 0.02lbs lb 200 2 2 A 0.0001in in Example: Find the pressure of a tire on pavement of a 10 ton truck with weight supported by 6 wheels each with 18 in2 in contact. Weight of truck = 10 tons = 10 tons x 2000lb = 20,000 lbs. 1ton The area of contact = 6 x 18 in2 = 108 in2. P F 20000lb lb 185.2 2 2 A 108in in Gauge Pressures vs Absolute Pressure Absolute pressure is the total amount of pressure. This includes the pressureof the atmosphere. If a gauge is used to measure the pressure, it will only tell you the pressure in the container that is bigger than atmospheric pressure. Air pressure is 14.7 lb/in2. Absolute Pressure = Gauge Pressure + Atmospheric Pressure Pabs PG Patmos or Example The gauge pressure of a tire is 30 lb/in2. What is the absolute pressure? Answer Absolute pressure is gauge pressure plus atmospheric pressure. 30 lb/in2 + 14.7 lb/in2 = 44.7 lb/in2 Example A car falls off a bridge and goes under water. The inside pressure is 15 lbs/in2. The outside pressure is 20 lbs in2. How hard must one push to open a car door which is 3 feet by 3 feet? Answer First find the area of the door in square inches. The door is 36 in by 36 in. Area = l x w = 36 in x 36 in = 1296 in2 Now for each square inch you must push with 5 lbs. Thus 5 lb/in2 x 1296 in2 = 6,480 lb. Therefore, you must push with 6,480 lbs of force. Is there a better way out of the car? 124 Pressure Increases with Depth As one goes deeper into a fluid the pressure increases in proportion to the depth. The pressure per square inch is equal to the weight of fluid over that square inch. Pressure does not depend on area of the container. Which dam must be stronger? The pressure can be calculated using the formula P D h P = D*h where D is the density and h is the height of the fluid. Example: A tank is filled to a depth of 18 feet with gasoline. What is the pressure on the side of the tank at the bottom? Answer: From the table in the density section, we find that the density of gasoline is 42.0 lb/ft3. The height of the fluid above the bottom of the tank is 18 feet. So the pressure is P D h 42.0 lb lb 18 ft 756 2 3 ft ft Notice that the units on the height cancelled with one of the units on the bottom of the density. Things work much smoother if the units are converted (if need be) to this this cancellation take place. This lets you have the right kind of units for the pressure (force units over area units) Example: How deep would you need to go in the ocean to find a pressure equal to the atmosphere at the surface (14.7 lb/in2)? First do the conversion from lb/in2 to lb/ft2. 14.7 lb 144in 2 lb 2116.8 2 2 2 in 1 ft ft Using the circle above and solving for h lb P ft 2 h 331 . ft lb D 64.0 3 ft 2116.8 125 Archimede’s Principle The buoyant force on an object in a fluid is equal to the weight of the displaced fluid. Example A hydrometer is put in battery acid to check the specific gravity (density) of the battery fluid. Which case represents the most fully charged battery? (Hint: A fully charged battery has the highest specific gravity.) Using the same hydrometer in all cases. If a fluid is dense, you will not need as much volume to equal the weight of the hydrometer. So the fluid where the hydrometer floats highest has the highest density. From this we would determine that the top battery is the one with the highest charge. 100 lbs 80 lbs In the above figure a rock is submerged in water. The buoyant force is 20 lbs. (100 lbs - 80 lbs) Thus the weight of water displaced is 20 lbs 126 From the definition of density, D V weight D Weight . The volume of water displaced by the rock is V 20lb 0.32 ft 3 . lb 62.4 3 ft The volume of the water displaced is equal to the volume of the rock because the rock is what displaces the water. Therefore we can use the above arrangement to find the volume of a weird shaped object. In this case Vobject = 0.32 ft3 But if we know the volume of the object and the weight of the object we can also determine the density of the object. D weight 100lb lb 307 3 3 V 0.32 ft ft 127 Pascal's Principle Pascal’s Principle states "A change in pressure at one point in a confined fluid is transmitted to all other points of the fluid." This principle is the basis for hydraulic equipment. Force out Force in -A -a The pressure is approximately the same at all points. Thus, the pressure on the big piston is the same as on the little piston, say 20 lbs/in2. If the area of the little piston, a, is 1 in2 and if the area of the large piston, A, is 100 in2 then the force at A is 100 times that at a. Fout , and from the formula for pressure, F=PA, so Fin A out . Ain Then the TMA TMA Fout PAout Fin PAin Sometime the information is given in terms of the diametrers of the pustons and not in terms of the areas. The formula for Area = 0.785 D2 , so when we add that to the equation for the TMA, 2 2 Aout 0.785 Dout Dout Thus TMA 2 . Ain 0.785 Din2 Din Therefore the TMA of hydraulic pressure is the ratio of the diameters squared. Example Find the TMA of the following hydraulic jack. TMAlever TMApressure 10in 10 1in (3in) 2 9 144 2 0.0625 (0.25in) Total TMA = 10 x 144 = 1440 Thus to lift 2880 lb object it only takes 2 lbs of force on the handle. 128 Bernoulli's Principle For the horizontal flow of a fluid through a tube, the sum of the pressure and energy of motion (kinetic energy) per unit volume of the fluid is constant, i.e., where the fluid moves fast the pressure is lower than where the fluid moves slow. Note a fluid is either a liquid or a gas. Examples of Bernoulli's Principle Low pressure a High pressure no flow. b. 129 Surface Tension The cohesive forces between water molecules can act as a kind of safety net for tiny objects (such as a needle or razor blade) placed on the surface of water. This is an example of the surface tension of water. Another example of the surface tension of water is the way water beads when placed on a glass plate (the cohesive forces of attraction between the water molecules pull them together into a spherical blob). The surface tension of water can be reduced by adding soap to the water. This reduced surface tension reduces the cohesion between the water molecules and thus makes it easier for the water to penetrate the fabric when washing clothes. The surface tension of most liquids decrease as the temperature increases. This is because the cohesive force of attraction is not as effective at hold the molecules together when they vibrate faster at higher temperatures. It is preferable for oil to have a fairly low surface tension so that it can spread evenly over the surfaces of the engine parts. Notice that if you place a drop of oil on a glass surface it does not bead up nearly as much as water does. It is also desirable to have an oil with a high enough surface tension to prevent oil film penetration. Viscosity The viscosity of a fluid (liquid or gas) is the measure of a fluids resistance to something traveling through it. Molasses has a high viscosity and rubbing alcohol has a low viscosity. The greater the molecular attraction between the liquid molecules the greater the viscosity. In addition the viscosity of a liquid also determines how long it will take a liquid to flow out of a hole in the bottom of a tank. We could thus say that viscosity is a measure of a fluids resistance to flow. The viscosity of a fluid normally decreases with increasing temperature. This is because as the temperature of a fluid increases the molecules vibrate more rapidly and hence the molecular forces of attraction are not as great. The viscosity of oil must be low enough to flow but high enough to resist oil film penetration. Multigrade (10W–40) oils have the characteristic that the viscosity of the oil does not vary nearly as much for a given temperature change as does a single grade oil (30W). Capillary Action The ability of water to climb up a small glass tube is an example of capillary action. The capillary action of water can be explained by the fact that the adhesive forces between water and glass are greater than the cohesive forces between the water molecules. Thus the water climbs up the glass until the cohesive forces between the water molecules can no longer support a higher column of water. The smaller the tube diameter the higher the water column can rise in the tube due to capillary action. This is easy to see since the weight of an 1 inch high column of water in a small diameter tube is much less than the weight of an 1 inch high column of water in a large diameter tube. The height that water rises in a capillary tube decreases with increasing temperature since the surface tension decreases with increasing temperature. 130 Important Functions of Oil 1. Provides protective film on metal surfaces to prevent metal to metal contact thus reducing heat generated by friction. 2. Transfers heat from inside the engine to the oil pan where it is given off to the surrounding air. 3. Cleans engine parts. Dirt and "crud" tend to settle to bottom of oil pan. 4. Provides a protective cushion between metal so as to absorb shock between bearings and other engine parts. 5. Forms a good seal between piston rings and the cylinder wall. Important Properties of Oil 1. 2. 3. 4. Proper viscosity over the expected temperature ranges i) low enough to flow ii) high enough to resist oil film penetration and to provide good sealing properties Surface Tension i) low enough to permit even spreading over metal ii) high enough to prevent oil film penetration Stability i) resist carbon formation, oxidation, foaming Detergency 131 Exercises Density Review 1. What is the density of a 150 g rock that occupies a volume of 60 cm3? D = ____________ 2. What is the volume of a piece of gold that has a weight of 1 lb? (the density of gold is 1223 lb/ft 3) V = _____________ 3. Water has a density of 62.4 lb/ft3 or 8.3 lb/gal. How many lbs of water can a 55 gal drum hold? Weight = _________________ 132 Pressure Review 1. A 150 lb lady stands on only one heel of her shoe. If the heel is square and has dimensions 1 of 4 in 41 in , what is the pressure that she exerts on the floor? P=___________ 2. Find the pressure at the bottom of a water filled drum that is filled 6.0 ft high? P=____________ 3. Find the depth in a lake at which the pressure would be 80.0 lb/in2. Hint First change 80 lb/in2 to units of lb/ft2. h=____________ 4. The weather channel reports that the atmospheric pressure is 28.5 in of mercury (28.5 in Hg) in central Nebraska. Find the atmospheric pressure in psi in Nebraska. P=_____________ 5. 20 psi is how many mm of Hg? P=_____________ 6. The atmospheric pressure at the surface of the earth is due to what? 7. What is the maximum height that water can be raised with a suction pump? 133 h=_____________ 8. Atmospheric pressure drops with altitude. There are several consequences of this. (a) How would this account for the lower temeprature at higher altitudes? (b) When cooking at higher altitudes, recipes often call for a pressure cooker, which is a sealed pot. Why would this be necessary to cook at temperatures near or above 100C ? 9. Jet aircraft require air to fly and routinely fly at altitudes of 30-40 thousand feet. If the atmospheric pressure was 14.7 psi all the way to the “top”, how deep would the atmosphere be? Would the jets be able to fly at their usual altitude? 134 Buoyancy Review 1. A balloon is buoyed up with a force equal to the a. weight of air it displaces. b. density of the surrounding air c. atmospheric pressure d. weight of the balloon and contents e. all of these 2. What is the weight of water displaced by a 100 ton floating ship? a. less than 100 tons b. 100 tons c. more than 100 tons d. 100 cubic feet e. depends on the ship's shape 3. Pumice is a volcanic rock that floats in water. Its density is a. less than the density of water. b. equal to the density of water. c. more than the density of water. 4. A metal alloy weighs 150 lbs in air and 120 lbs when completely submerged in water. What is the buoyant force acting on the object? FB = ______________ 5. What is the weight of water displaced by the object? What is the volume of water displaced by the object? What is the volume of the object Vwater=___________ Vobject=___________ 135 6. Find the density of the object. (from problems 4 and 5) D = _______________ 7. If a ship is in a closed lock and sinks will the water level be higher, lower, or at the same level on the sides of the lock compared to what it was before the ship sank? 136 Pascal's Principle Review P1 = P2 F1 F 2 or F1A2 = F2A1 A1 A2 1. The area of the small piston in a hydraulic jack is 1.5 in2 and the area of the large piston is 75 in2. If a force of 20 lb is applied to the small piston, what weight can be lifted by the large one? Fout = ___________ 137 Bernoulli's Principle Review 1. The faster a fluid moves, the a. greater its internal pressure. b. lower its internal pressure. 2. On a windy day, atmospheric pressure a. increases. b. decreases. c. remains unchanged. 3. with When water is turned on in a shower, the shower curtain moves towards the water. This has to do a. b. c. d. e. 4. capillary action. surface tension. heat capacity. pressure in a moving fluid. none of these. An umbrella tends to move upwards on a windy day because a. air gets trapped under the umbrella and pushes it up. b. buoyancy increases with increasing wind speed. c. a low pressure area is created on top of the umbrella. d. a and c above. e. all of these. 5. The Bernoulli effect causes passing ships to be drawn together when the ships are close and moving in the a. same direction. b. opposite direction. c. either the same direction or opposite direction. 6. Give an example of where Bernoulli's Principle is used in the gasoline engine. If you are unfamiliar with gasoline engines, explain how the Bernoulli effect works to let airplanes fly. 138 Surface Tension and Viscosity Review 1. The height that water raises in a capillary tube a. increases as the diameter of the tube increases. b. decreases as the diameter of the tube decreases. c. does not depend on the size of the tube. d. none of these. 2. The surface tension of water is caused by a. cohesive forces between water molecules. b. adhesive forces between water molecules. c. neither of these. 3. As the water temperature increases, the height water raises in a capillary tube a. decreases. b. increases. c. stays the same. 4. A consequence of surface tension of water is a. capillary action. b. wet sand being firmer than dry sand. c. hot oily soup tasting different than cold oily soup. d. all of the above. e. none of the above. 5. When you put a stick in water and remove it, the stick is wet. When you put a stick in mercury and remove it, the stick is dry. The reason for this is the adhesive forces are greater between the stick and a. water. b. mercury. c. not enough information given. 6. Is it possible for a solid piece of metal to float? Explain. 7. What is viscosity? 139 8. Give an example of a high viscosity liquid and a low viscosity liquid. High viscosity : ______________ Low Viscosity : ______________ 9. Does the viscosity of a liquid increase or decrease as the temperature increases? 10. Why will hot water leak more readily through small leaks in a car radiator than cold water? 140