Household Chemicals
advertisement

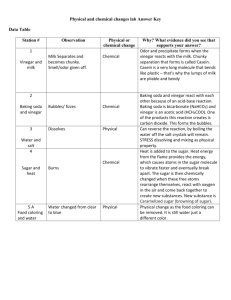
HOUSEHOLD CHEMISTRY Objectives: To make observations of chemical and physical changes involving household chemicals To identify the makeup of an unknown mixture containing two of these chemicals Discussion: Chemistry is the study of matter and the changes that matter can undergo. These changes can either be physical; where only the size, shape or state of a substance is altered, or chemical; which always results in the formation of one or more new substances, each with different compositions and chemical properties than the original substances. Visible indications that a chemical change has occurred can include a change in color, the formation of a solid when two solutions are mixed, or, in the formation of a gas. In this exercise, observations will be made regarding changes that may occur when four common household solids are mixed with three equally common, household liquids. These observations will then be used to identify the contents of an unknown mixture containing two of these of these solids. Procedure: 1. Place match-head sized samples of each of A A A the four household chemicals: A - baking soda, B B B B - flour, C - Alka Seltzer , D - sugar, in wells C C C of a clean, dry well plate as shown to the right: D D D 2. Using the hand lens, observe the physical properties of these four household chemicals. Record these properties onto the data page. 3. Add 10 drops of distilled water from the pipet labeled "water" to the baking soda ( A) in the first column of the well plate. Mix the contents of the well using the stirring rod (not the pipet tip). Record your observations onto the data table. Rinse off the stirring rod in a beaker of tap water. Repeat this procedure for the columns containing flour (B), Alka Seltzer (C), and sugar (D). rinsing off the stirring rod after use in each well. Record all of your observations onto the data table. 4. 5. Repeat steps # 3 and # 4, but this time use the pipet labeled "vinegar" (a 5 % solution of acetic acid and water) to add 10 drops of vinegar to each of the four substances in the second column. Again observe, and then record all of your observations onto the data table. 6. Use the "iodine" pipet to add 10 drops of iodine solution (iodine dissolved in water) to each of the four substances in the third column. Stir and mix, washing off the stirring rod after each well. CAUTION: IODINE SOLUTION CAN STAIN SKIN. Record your observations onto the data table. 7. Wash out and dry the well plate. The unknown is a mixture of two of the solids tested above. Copy the number of your unknown onto the data section. Place match-head sized samples of the unknown mixture into each of three wells in the first empty row across the well plate. Use the hand lens to describe the physical properties of your unknown. List your descriptions onto the data page. 8. Add 10 drops of water to the first well, 10 drops of vinegar to the second well and 10 drops of iodine solution to the third well. Stir and mix as before, washing off the stirring rod after use in each well. Record your observations onto the data table. Wash and dry the well plate, beaker and stirring rod. Name.................................................................. Partner................................................................ Lab Date............................ Chem. Cl............................ HOUSEHOLD CHEMISTRY Data: 1. Observations of the physical properties of the four household chemicals: A. Baking Soda......................................................................................................... B. Flour..................................................................................................................... C. Alka Seltzer ........................................................................................................ D. Sugar..................................................................................................................... 2. Describe the interactions of the four household chemicals with the different solutions: 3. Number of your unknown mixture .................. Observations of the physical properties of your unknown mixture .............................................. ........................................................................................................................................................ 4. Describe the interactions of your unknown mixture with the different solutions. Remember that your unknown exhibits the properties of BOTH of the two chemicals present. Continued On The Next Page Questions: 1. On the table below, identify which of the mixtures of household chemicals and solutions: a) Resulted in a CHEMICAL change (indicate by writing "C"). b) Identify which of the remaining samples resulted in only a PHYSICAL change (write "P"). c) Indicate in which of the combinations there were NO changes at all noticed (write "NONE"). 2. Two of the four household chemicals (baking soda, flour, Alka Seltzer , and sugar), were present in your unknown mixture. List which two you believe were present in your unknown. You MUST also indicate the reason for your answers. My unknown, # ............... , contained both ................................................. Reason why I feel this substance was in my unknown: and .................................................. my unknown: 3. be Reason why I feel this second substance was in Even when they were blended together in a mixture, both components in your unknown could identified. What characteristic of mixtures, which is not a property of compounds, allowed you to identify both substances present in your mixture ? HOUSEHOLD CHEMISTRY Per Pair of Students: 1- 12 well spot plate 1- 50 mL beaker vial labeled "baking soda" (powder) vial labeled "flour" vial labeled "Alka Seltzer" (powder, to resemble the other solids) vial labeled "sugar" vial labeled "unknown # ‘00’ " - two per table- unknowns consist of two of the four solids above; there are 6 different combinations; scoopulas plastic stirring rod hand lens bottle labeled "water" bottle labeled "vinegar" bottle labeled "iodine solution" (0.5 M I2 solution. Add 2.0 g KI to 40 mL of deionized water. Add 1.3 g of I2 and stir to dissolve, then dilute to 100 mL. Store in a dark bottle) 3 thin-stem pipets, labeled appropriately "water" , "vinegar" , or "iodine solution" UNKNOWNS 1. baking soda, alka seltzer baking soda, flour 7. flour, alka seltzer 2. baking soda, flour baking soda, alka seltzer 8. 3. baking soda, sugar flour, alka seltzer 9. 4. flour, alka seltzer alka seltzer, sugar 6. baking soda, alka seltzer soda, sugar alka seltzer, sugar 15. baking soda, sugar 11. 12. 14. flour, sugar 10. 5. flour, sugar baking soda, flour 13. 16. flour, sugar alka seltzer, sugar 17. 18. baking sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar sugar alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer alka seltzer flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour flour baking baking soda soda baking baking soda soda baking baking soda soda baking baking baking soda soda baking baking soda soda baking baking soda soda baking baking soda baking soda baking soda soda baking soda soda vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar vinegar distilled distilled water water distilled distilled water water distilled distilled distilled water water distilled distilled water water distilled distilled distilled water water distilled distilled water water distilled iodine iodine solution solution iodine iodine solution solution water water water iodine solution distilled distilled water distilled water distilled water distilled water iodine solution distilled distilled water distilled water distilled distilled water distilled water iodine solution water water distilled water iodine solution water iodine solution iodine iodine solution solution iodine iodine solution solution iodine iodine solution solution iodine iodine solution solution iodine solution iodine solution iodine solution iodine solution iodine solution iodine solution iodine solution iodine solution iodine solution iodine solution