Advances in Peritoneal Dialysis, Vol. 26, 2010 1,2 Brenda Wiggins
advertisement

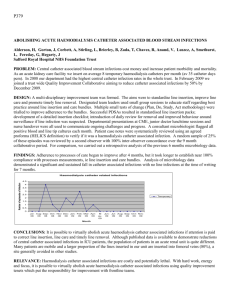
A dv an ce s in P eri to ne al Di al ys is, V ol. 26 , 20 10 1,2 1 Yijuan Brenda Wiggins, 3,4 Sun,1,2 1,2 5, 6 Imaging of Peritoneal Catheter Tunnel Infection Using Positron-Emission Tomography Pooja Singh,1,2Reuben E. Last, Michael F. Hartshorne, Karen S. Servilla, Antonios H. Tzamaloukas Imaging by ultrasonography or scintigraphy may assist in the diagnosis and management of tunnel infections of the peritoneal dialysis (PD) catheter. Here, we report a case of tunnel infection in which imaging with positron-emission tomography (PET) correctly predicted failure of conservative management. A 61-year-old man with diabetic nephropathy commenced PD in January 2008. He developed erythema and drainage at the exit site, with negative cultures in February 2008, and frank exit-site infection (ESI) with purulent drainage growing methicillinsensitive Staphylococcus aureus [MSSA (treated with 3 weeks of oral dicloxacillin)] in August 2008. Subsequently, MSSA-growing purulent drainage from the exit site persisted. Systemic antibiotics were not administered, but there was gradual improvement with gentamicin ointment alone. In November 2008, the patient developed partial extrusion of the outer cuff of the PD catheter. In January 2009, a new ESI developed. Despite a week of treatment with cefazolin and gentamicin, the patient still developed his first episode of peritonitis with coagulase-negative Staphylococcus. He then received intraperitoneal vancomycin with good response. Although the ESI appeared to have responded to the treatment, PET imaging showed increased fludeoxyglucose (FDG) activity in the whole abdominal wall portion of the PD catheter. The patient resisted removal of the catheter and had no further signs of infection until June 2009. At that time he presented with exuberant inflammatory tissue (“proud 1From: 2Renal Section, Raymond G. Murphy Veterans Affairs Medical Center (RGM VAMC); 34Surgery Service, RGN VAMC; Division of Nephrology, University of New Mexico School of Medicine (UNM SOM); 5Department of Surgery, UNM SOM; 6Radiology Service, RGM VAMC; and Department of Radiology, UNM SOM, Albuquerque, New Mexico, U.S.A. flesh”) at the exit site. Repeated PET imaging again showed increased FDG activity along the abdominal wall portion of the catheter. The PD catheter was removed and found to be infected. The patient was placed on temporary hemodialysis. This case demonstrates that PET imaging, in addition to other imaging techniques, may be useful for diagnosing and managing PD catheter infections. Key words Exit-site infection, tunnel infection, positron-emission tomography, tunnel imaging Introduction Exit-site infections (ESIs) and tunnel infections of the peritoneal dialysis (PD) catheter are ongoing problems. Exit-site infections are routinely diagnosed by clinical examination (1). Tunnel infections usually occur in the presence of ESIs. Staphylococcus aureus and Pseudomonas aeruginosa ESIs are often associated with concomitant tunnel infections. However, tunnel infections may also occur in the absence of clinically recognized ESIs (2). The presence and the extent of a tunnel infection both affect the management of the infected patient. Peritonitis occurring in a patient with tunnel infection does not usually respond to antibiotic therapy without catheter removal (3). Tunnel infections without concomitant peritonitis can be treated with limited surgical intervention and salvage of the infected catheter (4). Imaging techniques may be of help in diagnosing tunnel infections (5). Imaging procedures address two questions critical for planning the management of an infection: • Is a tunnel infection present? • What is the extent of the tunnel infection along the length of the portion of the catheter within the abdominal wall? Singh et al. 97 Ultrasonography and scintigraphy with labeled pain, and again visited the outside hospital in his home town. Blood and PD fluid cultures were 18white blood cells (WBCs) have been used for obtained at the outside facility, and he was given imaging tunnel infections. Here, we present a 1 dose of intravenous vancomycin. He was then case of tunnel infection in which transferred to our hospital for further care. positron-emission tomography (PET) with At presentation, the patient was afebrile with a F-fluorodeoxyglucose (FDG) was helpful in blood pressure of 150/82 mmHg and a pulse rate defining the management goals. of 81. Case Report A 61-year-old man with diabetic nephropathy, who had been on PD for 1 year, was admitted for treatment of peritonitis in January 2009. Before this admission and since PD catheter placement in December 2007, this patient had had a long history of ESIs. Initially, the exit site lacked any signs of infection. In February 2008, the patient developed erythema and purulent drainage from the exit site. This ESI was treated with 1 g intravenous vancomycin and a 7-day course of oral cephalexin. Exit-site cultures revealed no growth. In August 2008, purulent drainage from the exit site recurred. Cultures from the site grew methicillinsensitive Staphylococcus aureus (MSSA). The patient did not have any evidence of peritonitis at that time. He was treated with dicloxacillin for a total of 3 weeks. After completion of the course of dicloxacillin, the exit site continued to be erythematous, with open bleeding areas around the site and continuous purulent drainage. New cultures from the exit site again grew MSSA. At that time, antibiotics were not restarted, but continued care of the exit site with gentamicin ointment was maintained. In a follow-up visit 1 month later, the exit site appeared to be healing, with improvement in erythema. In November 2008, mild protrusion of the external cuff was noted. The exit site appeared dry and without signs of infection at that time. In January 2009, the patient was admitted with his first episode of peritonitis. Seven days before the admission, he reported erythema around the exit site. Intraperitoneal cefazolin and gentamicin were started at an outside hospital. Exit-site cultures from the outside hospital grew MSSA. On the last day of treatment for the ESI, the patient noted cloudy PD fluid and abdominal His abdomen was soft, non-distended, with normoactive bowel sounds and mild tenderness to palpation of the right upper and lower quadrants. The exit site was erythematous and indurated, but without drainage. Of 3 blood cultures from the outside hospital, 2 cultures—and the PD fluid culture—grew coagulasenegative Staphylococcus. Repeat blood cultures at our hospital were negative. The patient was subsequently started on intraperitoneal vancomycin and was treated for 2 weeks. An FDG PET scan of the patient’s abdomen disclosed increased FDG activity along the length of the PD catheter tunnel, including both cuffs [Figure 1(A)]. No evidence of peritonitis was present, but the patient had already received several doses of antibiotics at the time that the imaging study was performed. Removal of the PD catheter was advised. However, the patient, who lives far away from the dialysis unit, declined to consent to catheter removal. Together, the patient and the nephrology team determined that the catheter would continue to be used for PD, but that it would have to be removed if the patient developed another episode of overt catheter-related infection. At a subsequent follow up, that patient showed no signs of ESI or peritonitis. In March 2009, no drainage or erythema was observed around the exit site. Furthermore, the outer cuff no longer appeared to be exposed. In May 2009, pus was expressed from the patient’s exit site during a routine PD clinic visit. Cultures from the PD fluid were negative, but the exit-site culture grew MSSA. Thus, the patient was treated with oral cephalexin for 2 weeks. The ESI subsequently resolved after completion of antibiotics. In June 2009, the patient reported cloudy fluid draining from his PD catheter. The exit site was noted to be indurated and erythematous; it also had evidence of new exuberant tissue (“proud flesh”) growth, indicating a chronic infection. The outer cuff was also fully exposed. The patient was readmitted and started on intravenous vancomycin. The exit site grew MSSA, and the PD fluid contained WBCs (205 cells/mL), but did not grow any organism. Repeated FDG PET imaging showed recurrent or persistent tunnel infection and areas of inflammation through the abdominal wall at each end of the tunnel [Figure 1(B)]. The PD catheter was removed and, on inspection, found to be grossly infected. The patient was treated for 3 weeks with intravenous vancomycin and put on hemodialysis. A 98 Imaging of PD Catheter Tunnel Infection 1 Positron-emission tomography images showing increased activity along the abdominal wall portion of the peritoneal dialysis catheter. Arrows point to inner cuff. (A) January 2009. (B) June 2009. FIGURE Tunnel infections produce widening and loss of clarity of the circumferential rim. This area new PD catheter was placed in November 2009 becomes hypoechogenic, showing fluid after complete healing of the infected abdominal collection (10,11). Abscesses can produce wound. larger areas of reduced echotexture. These areas may be similar to those seen with Discussion The management of PD catheter cellulitis, but by compression with the infections involves one of three methods: ultrasound transducer, an abscess will be deformable and compressible (2). • conservative management with antibiotics Scintigrams using WBCs labeled either with alone, • management with antibiotics plus In111 oxine (12) or with Tc-99m exametazime minimal surgical (13) have also been used to image infected PD intervention (for example, shaving the outer catheter tunnels. The use of scintigraphy cuff) aimed at saving the catheter, or • management with antibiotics and removal of the confirmed that previously reported failures in saving infected PD catheters by shaving the catheter. outer cuff (14) were routinely associated with To decide on the appropriate management, infection of the inner cuff (15). any question about the presence or the extent of Imaging by FDG PET has been extensively used in diagnosing and staging tumors. Its tunnel infection should be cleared. Imaging second widespread use is in the diagnosis of should be considered if there is uncertainty deep-seated infections. Imaging by FDG PET about either of these two issues. has proved to helpful in the diagnosis of infected foreign material in the body (such as Ultrasonography has been the imaging modality prosthetic cardiac valves, pacemaker wires, most commonly employed for diagnosis of PD prosthetic joints, aortic grafts, and central catheter infections (6–9). A low-echo venous catheters) and of occult infections in circumferential rim is seen around the catheter hemodialysis under normal circumstances (5). Singh et al. 99 vascular access (16). The case presented here illustrates tunnel infections was around 70%; the specificity was 100% (18). In another report, a labeled WBC the use of FDG PET imaging in PD catheter scan had a sensitivity of 83% and a specificity of infections. In the presence of several imaging 75% in diagnosing PD catheter infection (13). An methods for PD advantage of labeled WBC scans is that they catheter infections, the choice of method is determine the extent of infection along the length routinely based on 5 criteria: of the catheter with accuracy, and they are • Availability • helpful in the decision to remove the PD catheter Local expertise • (15). Risks • Performance • Costs The differences between the three techniques are small for the first 3 criteria. Ultrasonography is, in general, available to many institutions at which PD patients are followed. Scintigraphy and FDG PET imaging are less available. A well-trained radiologist and a radiology department with expertise in the relevant technique are indispensable for avoiding diagnostic errors regardless of the technique used. Ultrasonography has no recognizable risks ; scintigraphy and FDG PET imaging both have a small risk from exposure to radiation. The three imaging methods differ substantially in the two last criteria mentioned, however. Despite the larger number of reported studies, the accuracy of ultrasonography in diagnosing PD catheter infections has not been adequately studied. Looking at studies of ultrasonography in other deep-seated infections, it appears that the accuracy of the procedure is not great. For example, one report indicated that the sensitivity of ultrasound in detecting abdominal abscess in patients after colorectal surgery was only 43%; the specificity was 100%, and the overall accuracy was 65% (17). In addition to its low sensitivity, ultrasonography is poor in detecting the extent of deep infections. Labeled WBC scintigrams may show collections of pus deep in the catheter tunnel in cases in which ultrasonography fails to demonstrate any abnormality around the peritoneal catheter (18). In that report, the sensitivity of In-111 WBC scans in detecting deep Although the accuracy of FDG PET in diagnosing recommendations. Perit Dial Bull 1987;7:130–8. tunnel infections of the PD catheter will need to be evaluated in future studies, the procedure has been shown to have a high diagnostic accuracy for deep abscesses. In one study of 165 infections in 148 of 248 patients with multiple myeloma, FDG PET identified the site of the infection in 46 episodes in which other imaging methods failed (19). In a meta-analysis of infections in prosthetic joints, the pooled sensitivity of FDG PET in diagnosing those infections was 82.1%; the specificity was 86.6% (20). In the case reported here, FDG PET imaging was accurate both in diagnosing the tunnel infection and in determining its extent. The costs of imaging procedures vary by geographic area in the United States. In New Mexico, Medicare pays $121.24 for ultrasonography, $1065.54 for WBC scans, and $1350.82 for FDG PET scans with computed tomograms. Thus the choice of imaging technique for a tunnel infection of the PD catheter appears to be between a cheaper but relatively inaccurate method (ultrasonography) and one of two accurate but expensive methods (scintigraphy, FDG PET). Conclusions Imaging by FDG PET is an expensive but accurate method of diagnosing deep-seated infection and seems to be useful in tunnel infections of the PD catheter. The case presented here demonstrates that FDG PET imaging can be used to diagnose the presence and extent of PD catheter-related tunnel infections and to guide their treatment. Acknowledgments This work was supported by the Raymond G. Murphy VA Medical Center. The authors thank Mr. James Jannis of the Department of the Medical Media at that institution for his invaluable assistance. References 1 Twardowski ZJ, Prowant BF. Classification of normal and diseased exit sites. Perit Dial Int 1996;16(Suppl 1):S32–50. 2 Piraino B. Management of catheter related infections. Am J Kidney Dis 1996;27:714–18. 3 Piraino B, Bailie GR, Bernardini J, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005;25:107–31. 4 Oreopoulos DG, Baird Helfrich G, Khanna R, et al. Peritoneal catheter and exit-site practices: current 100 Imaging of PD Catheter Tunnel Infection 5 Taylor PM. Image-guided peritoneal access and management of complications in peritoneal dialysis. Semin Dial 2002;15:50–8. 6 Scanziani R, Pozzi M, Pisano L, et al. Imaging work-up for peritoneal access care and peritoneal dialysis complications. Int J Artif Organs 2006;29:142–52. 7 Plum J, Sudkamp S, Grabensee B. Results of ultrasound-assisted diagnosis of tunnel infections in continuous peritoneal dialysis. Am J Kidney Dis 1994;23:99–104. 8 Korzets Z, Erdberg A, Golan E, et al. Frequent involvement of the internal cuff segment in CAPD patients and exit-site infection—an ultrasound study. Nephrol Dial Transplant 1996;11:336–9. 9 Vychytil A, Lorenz M, Schneider B, Hörl WH, Haag– Weber M. New criteria for management of catheter infections in peritoneal dialysis patients using ultrasonography. J Am Soc Nephrol 1998;9:290–6. 10 Thodis E, Passadakis P, Ossareh S, Panagoutsos S, Vargemezis V, Oreopoulos DG. Peritoneal catheter exit-site infections: predisposing factors, prevention and treatment. Int J Artif Organs 2009;26:698–714. 11 Keane WF, Bailie GR, Boeschoten E, et al. Adult peritoneal dialysis–related peritonitis treatment recommendations: 2000 update. Perit Dial Int 2000;20:396–411. 12 Kipper SL, Steiner RW, Wilztum KF, et al. In-111– leukocyte scintigraphy for detection of infection associated with peritoneal dialysis catheters. Radiology 1984;151:491–4. 99m13 Ruiz Solis S, Garcia Vicente A, Rodaco Marina S, et al. Diagnosis of the infectious complications of continuous ambulatory peritoneal dialysis by TcHMPAO labeled leucocytes [Spanish]. Rev Esp Med Nucl 2004;23:403–13. 14 Piraino B, Bernardini J, Peitzman A, Sorkin M. Failure of peritoneal catheter cuff shaving to eradicate infection. Perit Dial Bull 1987;7:179–82. 15 Gibel LJ, Quintana BJ, Tzamaloukas AH, Garcia DL. Soft tissue complications of Tenckhoff catheters. Adv Perit Dial 1989;5:229–33. 16 Carlos MG, Juliana R, Matilde N, et al. Hidden clotted vascular access infection diagnosed by fluorodeoxyglucose positron emission tomography. Nephrology (Carlton) 2008;13:264–5. 17 Lin CM, Hung GU, Chao TH, Lin WY, Wang SJ. The limited use of ultrasound in the detection of abdominal abscesses in patients after colorectal surgery: compared with gallium scan and computed tomography. Hepatogastroenterology 2005;52:79–81. 18 Gibel LJ, Hartshorne MF, Tzamaloukas AH. Indium111 oxine leukocyte scan in the diagnosis of peritoneal catheter tunnel infections. Perit Dial Int 1998;18:234–5. 1819 Mahfouz T, Miceli MH, Saghafifar F, et al. FFluorodeoxyglucose positron emission tomography contributes to the diagnosis and management of infections in patients with multiple myeloma: a study of 165 infectious episodes. J Clin Oncol 2005;23:7857–63. 20 Kwee TC, Kwee RM, Alavi A. FDG-PET for diagnosing prosthetic joint infection: systematic review and metaanalysis. Eur J Nucl Med Mol Imaging 2008;35:2122–32. Corresponding author: Pooja Singh, MD, 1 University of New Mexico, MSC 10-5550, Albuquerque, NM 87131 U.S.A. E-mail: psingh@salud.unm.edu