Detecting Atmospheric Carbon Dioxide
advertisement

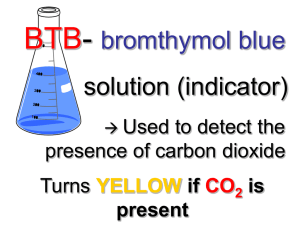
STEM Detecting Atmospheric Carbon Dioxide This activity is adapted from the LHS GEMS book, “Global Warming & the Greenhouse Effect” published by the Lawrence Hall of Science at the University of California at Berkeley in 1990. The STEM Context A study of the concentration of carbon dioxide gas in air samples requires an understanding of effective sampling techniques, the use of pH indicators, an understanding of chemical reactions that result on the formation of acidic compounds, and an understanding of the chemical processes that contribute to the composition and characteristics of Earth’s atmosphere. Examples of Applicable National Learning Standards: As a result of activities in Grades 5-8, all students should develop an understanding of properties and changes in properties in matter. Content Standard B; The National Science Education Standards; Page 149. One example of a fundamental concept and principle that underlies that standard is: “Substances react chemically in characteristic ways with other substances to form new substances (compounds) with different characteristic properties.” Page 154 As a result of activities in Grades 9-12, all students should develop an understanding of chemical reactions. Content Standard B; The National Science Education Standards; Page 176. One example of a fundamental concept and principle that underlies that standard is: “Reaction rates depend on how often the reacting atoms and molecules one another, on the temperature, and on the properties – including shape- of the reacting species.” Page 179 Procedural Issues Students should be reminded to bubble the air sample through the BTB solution to maximize the opportunity for CO2 molecules to react with water molecules. The collection of an air sample from a car may require that an adult collect the sample for students to analyze. www.umassk12.net/ipy A STEM ED Program at the University of Massachusetts, funded by the National Science Foundation and supported by the Climate System Research Center in conjunction with the International Polar Year Bromothymol blue (BTB) Source: http://en.wikipedia.org/wiki/Bromothymol_blue The WikipediA web site is one example of many web sites that provide information about the use of bromothymol blue as an indicator of a small change in the pH of a solution BTB indicator solutions have a blue color when the pH of the BTB solution is slightly higher than 7.0. As the pH of a BTB decreases, the color of the solution changes from blue to yellow as the pH changes from 7.6 to 7.0 and then to 6.0. The use of a BTB solution to analyze air sample provides an opportunity to discuss the characteristics of weak and strong acids as well as the reversibility of many chemical reactions as is the case with the formation of carbonic acid. CO2 + H2O ↔ H2CO3 Sample Answers to Questions The calculation of the mass percent concentration of the more dilute BTB solution provides an opportunity to discuss solution concentrations that include molarity, normality, molality, and mass percent concentration. The expression of the mass percent concentration of the more dilute BTB solution also provides an opportunity to remind students of the general rules associated with significant figures. Examples of Answers to Questions Question 1: Equal volumes of air samples will be collected. The rate at which the air sample is bubbled through the BTB solution will be the same of all samples. The air sample will be bubbled through the solution slowly to maximize the interaction between carbon dioxide and water. Question 2: 131.98 g − 126.95 g = 5.03 grams of BTB solution Question 2: 3.03 g x 0.0004 = 0.002 grams of BTB solute Question 3: 182.04 g − 126.95 g = 55.09 grams of a more dilute BTB solution Question 4: 0.002 grams of BTB ÷ 55.09 grams of solution x 100 = 0.0036% Question 5: The accuracy of mass and volume measurements will influence the accuracy of the calculation. .