Complete Blood Count
advertisement
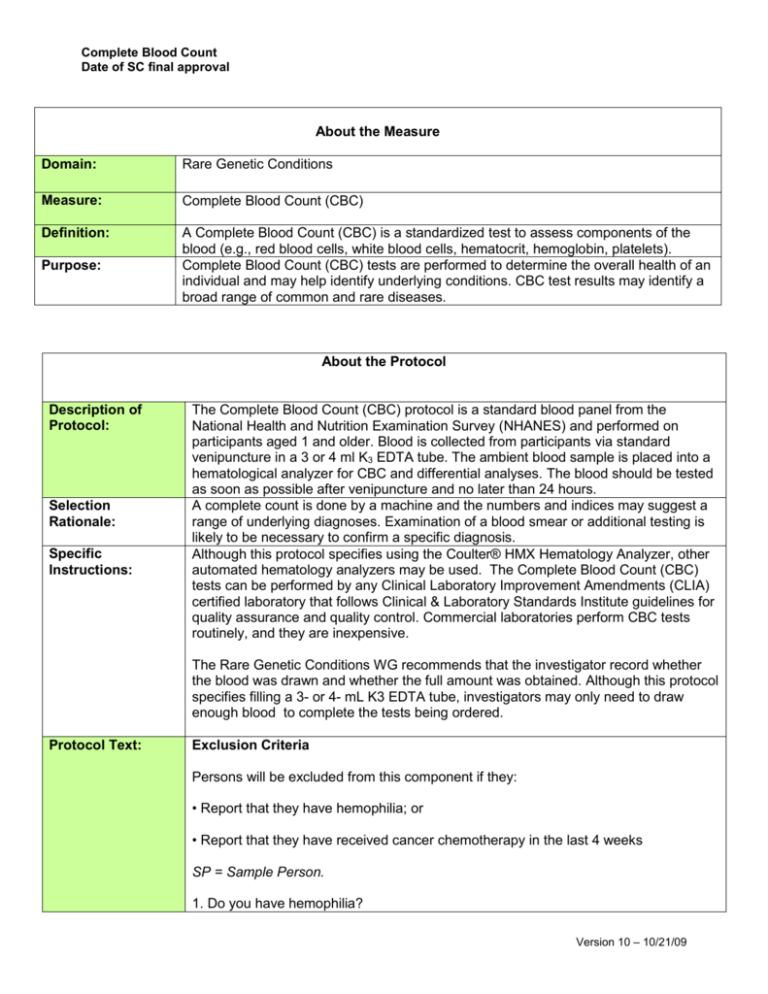
Complete Blood Count Date of SC final approval About the Measure Domain: Rare Genetic Conditions Measure: Complete Blood Count (CBC) Definition: A Complete Blood Count (CBC) is a standardized test to assess components of the blood (e.g., red blood cells, white blood cells, hematocrit, hemoglobin, platelets). Complete Blood Count (CBC) tests are performed to determine the overall health of an individual and may help identify underlying conditions. CBC test results may identify a broad range of common and rare diseases. Purpose: About the Protocol Description of Protocol: Selection Rationale: Specific Instructions: The Complete Blood Count (CBC) protocol is a standard blood panel from the National Health and Nutrition Examination Survey (NHANES) and performed on participants aged 1 and older. Blood is collected from participants via standard venipuncture in a 3 or 4 ml K3 EDTA tube. The ambient blood sample is placed into a hematological analyzer for CBC and differential analyses. The blood should be tested as soon as possible after venipuncture and no later than 24 hours. A complete count is done by a machine and the numbers and indices may suggest a range of underlying diagnoses. Examination of a blood smear or additional testing is likely to be necessary to confirm a specific diagnosis. Although this protocol specifies using the Coulter® HMX Hematology Analyzer, other automated hematology analyzers may be used. The Complete Blood Count (CBC) tests can be performed by any Clinical Laboratory Improvement Amendments (CLIA) certified laboratory that follows Clinical & Laboratory Standards Institute guidelines for quality assurance and quality control. Commercial laboratories perform CBC tests routinely, and they are inexpensive. The Rare Genetic Conditions WG recommends that the investigator record whether the blood was drawn and whether the full amount was obtained. Although this protocol specifies filling a 3- or 4- mL K3 EDTA tube, investigators may only need to draw enough blood to complete the tests being ordered. Protocol Text: Exclusion Criteria Persons will be excluded from this component if they: • Report that they have hemophilia; or • Report that they have received cancer chemotherapy in the last 4 weeks SP = Sample Person. 1. Do you have hemophilia? Version 10 – 10/21/09 Complete Blood Count Date of SC final approval 1[] 2[] 7[] 9[] Yes No Refused Don’t Know If the SP answers, "Yes," the SP is excluded from the blood draw. If SP answer "No" or "Don’t Know," blood is drawn from the SP. 2. Have you received cancer chemotherapy in the past four weeks or do you anticipate such therapy in the next four weeks? 1[] 2[] 7[] 9[] Yes No Refused Don’t Know If the SP answers, "Yes," the SP is excluded from the blood draw. If SP answer "No" or "Don’t Know," blood is drawn from the SP. Venipuncture Procedures Editor’s Note: Please review chapter 4 of the Laboratory Procedures Manual from the 2009–2010 National Health and Nutrition Examination Survey for a full description of Phlebotomy procedures. Venipuncture should generally be performed using the median cubital, cephalic, or basilic veins in the left arm unless this arm is unsuitable. If the veins in the left arm are unsuitable, look for suitable veins on the right arm. If the veins in the antecubital space on both arms are not suitable, then look for veins in the forearm or dorsal side of the hand on the left arm/hand and then the right arm/hand. Fill a 3 or 4 ml K3 EDTA tube with blood. Recording the Results of the Venipuncture Procedure Immediately after completing the venipuncture, record the results of the blood draw, the reasons for a tube not being drawn according to the protocol, and any comments about the venipuncture. Perform CBC Analyses There are a number of different assays and Clinical Laboratory Improvement Amendments (CLIA) certified instruments that are appropriate to perform the CBC analyses. Once an assay is chosen for a particular study, the Working Group recommends that no changes in the protocol be made over the course of the study. To aid comparability, the Working Group recommends that the investigator record the make and manufacturer of equipment used and the repeatability and coefficients of variation for the assay. Version 10 – 10/21/09 Complete Blood Count Date of SC final approval Note: a full description of the NHANES procedure is found in the 2009–2010 NHANES Lab Procedures Manual. If the Coulter® HMX Hematology Analyzer is utilized, it should be calibrated per the manufacturer’s recommendations. NHANES performs the test in duplicate, but this may not be necessary. The following parameters and units are measured in the CBC panel test. White blood cell count (1,000 cells/uL) Lymphocyte (%) Monocyte (%) Segmented neutrophils (%) Eosinophils (%) Basophils (%) Lymphocyte number (1,000 cells/uL) Monocyte number (1,000 cells/uL) Segmented neutrophils number (1,000 cells/uL) Eosinophils number (1,000 cells/uL) Basophils number (1,000 cells/uL) Red cell count (million cells/uL) Hemoglobin (g/dL) Hematocrit (%) Mean cell volume (fL) Mean cell hemoglobin (pg) MCHC (g/dL) Red cell distribution width (%) Platelet count (1,000 cells/uL) Mean platelet volume (fL) Interpretation of the CBC Results Participant: The 2009–2010 NHANES Laboratory Procedures Manual includes several tables of normal ranges for the CBC tests, see Chapter 7, pg 125. Age 1 and older Source: Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). (2010). Complete Blood Count. In Laboratory procedures manual (pp. 7-3–7-130). Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Language of Source: English Personnel and Training Required: Phlebotomist Laboratory that can perform Complete Blood Count (CBC) test Equipment Needs: Phlebotomy supplies Version 10 – 10/21/09 Complete Blood Count Date of SC final approval Protocol Type: Bioassay Requirements: Requirements Category Common Data Elements: General References: Required (Yes/No): Major equipment No Specialized training No Specialized requirements for biospecimen collection Average time of greater than 15 minutes in an unaffected individual No No Cheng, C. K., Chan, J., Cembrowski, G. S., & van Assendelft, O. W. (2004). Complete blood count reference interval diagrams derived from NHANES III: Stratification by age, sex, and race. Laboratory Hematology, 10(1), 42–53. Lim, E. M., Cembrowski, G., Cembrowski, M., & Clarke, G. (2010). Race-specific WBC and neutrophil count reference intervals. International Journal of Laboratory Hematology, 32(6 Pt. 2), 590–597. Additional Information About the Measure Essential Data: Current Age Related PhenX Measures: Derived Variables: Lipid Profile Keywords/Related Concepts: Complete blood count, CBC, red blood cells, white blood cells, hemoglobin, hematocrit, eosinophils, basophils, platelets, National Health and Nutrition Examination Survey, NHANES None Version 10 – 10/21/09