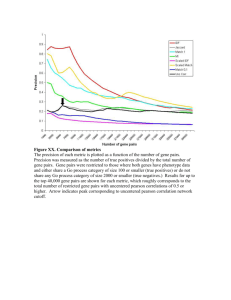
Supplementary Table 3: Comparison of size variation, efficacy and
advertisement

Supplementary Table 3: Comparison of size variation, efficacy and induction response of synthetic and native promoters Serial Number Name of Promoter 1 4×W2 Size of promot er (bp) 112 Sources of promoter Size of Native Promoter 2 4×W1 120 PR1 Gene Parsley 840 bp 3 4 ×D 124 PR2 Gene Parsley ------- PR1 Gene Parsley 840 bp Induction Condition Reference (Rushton et al. 1996) 4 4×S 96 ELI7.1 Gene Parsley ELI7.2 Gene Parsley 5 4 × GST 100 bp GST 1 Potato 6 EE/ FF CaMV35S 7 FFEE CaMV35S 8 C18 49 bp HVA1 Gene 68 bp pathogenderived peptide elicitor, pep25 pathogenderived peptide elicitor, pep25 pathogenderived peptide elicitor, pep25 pathogenderived peptide elicitor, pep25 pathogenderived peptide elicitor, pep25 Rhizoctonia solani Sclerotina sclerotioru m 20μM ABA 9 C19 49 bp HVA1 Gene 68 bp 20μM ABA 10 C20 49 bp HVA1 Gene 68 bp 20μM ABA 11 C21 49 bp HVA1 Gene 68 bp 20μM ABA 12 C22 51 bp HVA1 Gene 68 bp 20μM ABA 380 bp 1400bp (Rushton et al. 1996) (Rushton et al. 1996) (Kirsch et al. 2000) (Strittmatter et al. 1996) (Kirsch et al. 2001) (Heise et al. 2002) (Hong et al. 1988) (Hong et al. 1988) (Hong et al. 1988) (Hong et al. 1988) (Hong et al. 13 GT1NOS101 165 bp pea rbcS-3A promoter and Nopaline synthase gene 410 bp 14 Z-NOS101 147 bp 1120 bp 15 G-NOS101 153 bp Z-motif of the Arabidopsis cab1 promoter and Nopaline synthase gene Arabidopsis cab promoter and Nopaline synthase gene 16 GATANOS101 149 bp 1721 bp 17 G/GATANOS101 197 bp Arabidopsis rbcS promoter and Nopaline synthase gene ----- 18 GT1/GATANOS101 209 bp ------ ------ 19 4H1-46 21 bp Histone gene promoter 681 bp 20 mPtDrl02 1041 bp PtDrl02 promoter derived from poplar [(Populus tomentosa x P. bolleana) x P. tomentosa] 986 bp 21 Triple-Op 117 bp one operator (O1)- 1 bp up- stream of the TATA-box, a second operator (O2)- 1 bp downstream of the 350 bp 950 bp ----- white light (100 mmol/m2/s) and blue light white light (100 mmol/m2/s) 1988) (Mitra and An 1989) (Mitra and An 1989) white light (100 mmol/m2/s) and blue light white light (100 mmol/m2/s) (Mitra and An 1989) Far red light, red light and blue light Far red light, red light and blue light (NaCl, 0.25 M), ABA (100 pm ) 2,4-D (2.40, 100 pm) and 6-BAP (100 pm) Methyl jasmonate (200 μM) (Mitra and An 1989) Tetracycline (0.1 mg/l) (Gatz et al. 1991) (Mitra and An 1989) (Mitra and An 1989) (Mikami et al. 1987) (Zheng et al. 2010) 22 pCAR46C (4 x 38 bp =152 bp) 23 6 x XRE-P 109 bp 24 4xNRE-minGUS 172 bp 25 DR5(8×) 88 bp TATA-box and a third operator (O3)- 23 bp downstream of the TATA-box fused to CaMV 35 S promoter Chalcone synthase (chs15) promoter 277 bp six tandem repeated XRE sequences of CYP1A1 gene Nitrite reductase (NIR1) promoter 3.1 kb AuxRE from the GH3 promoter 592 bp 3.1 kb p-coumaric acid (5 x 10-4 M) 3-methylCholanthren e (5 nM) (Ryder et al. 1987) 10 mM KNO3 and 10 mM NH4NO3 αnaphthalene acetic acid (50 μM) (Konishi and Yanagisawa 2010) (Xu and Pasco 1998) (Liu et al. 1994) References: Gatz C, Kaiser A, Wendenburg R (1991) Regulation of a modified CaMV 35S promoter by the Tn10-encoded Tet repressor in transgenic tobacco. Molecular & general genetics : MGG 227: 229-237 Heise A, Lippok B, Kirsch C, Hahlbrock K (2002) Two immediate-early pathogen-responsive members of the AtCMPG gene family in Arabidopsis thaliana and the W-box-containing elicitorresponse element of AtCMPG1. Proceedings of the National Academy of Sciences of the United States of America 99: 9049-9054 Hong B, Uknes SJ, Ho TH (1988) Cloning and characterization of a cDNA encoding a mRNA rapidly-induced by ABA in barley aleurone layers. Plant molecular biology 11: 495-506 Kirsch C, Takamiya-Wik M, Schmelzer E, Hahlbrock K, Somssich IE (2000) A novel regulatory element involved in rapid activation of parsley ELI7 gene family members by fungal elicitor or pathogen infection. Molecular plant pathology 1: 243-251 Kirsch C, Logemann E, Lippok B, Schmelzer E, Hahlbrock K (2001) A highly specific pathogen-esponsive promoter element from the immediate-early activated CMPG1 gene in Petroselinum crispum. The Plant journal : for cell and molecular biology 26: 217-227 Konishi M, Yanagisawa S (2010) Identification of a nitrate-responsive cis-element in the Arabidopsis NIR1 promoter defines the presence of multiple cis-regulatory elements for nitrogen response. The Plant journal : for cell and molecular biology 63: 269-282 Liu ZB, Ulmasov T, Shi X, Hagen G, Guilfoyle TJ (1994). Soybean GH3 promoter contains multiple auxin-inducible elements. Plant Cell 6, 645-657. Mikami K, Tabata T, Kawata T, Nakayama T, Iwabuchi M (1987) Nuclear protein(s) binding to the conserved DNA hexameric sequence postulated to regulate transcription of wheat histone genes. FEBS letters 223: 273-278 Mitra A, An G (1989) Three distinct regulatory elements comprise the upstream promoter region of the nopaline synthase gene. Molecular & general genetics : MGG 215: 294-299 Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich IE (1996) Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. The EMBO journal 15: 5690-5700 Ryder TB, Hedrick SA, Bell JN, Liang XW, Clouse SD, Lamb CJ (1987) Organization and differential activation of a gene family encoding the plant defense enzyme chalcone synthase in Phaseolus vulgaris. Molecular & general genetics : MGG 210: 219-233 Strittmatter G, Gheysen G, Gianinazzi-Pearson V, Hahn K, Niebel A, Rohde W, Tacke E (1996) Infections with various types of organisms stimulate transcription from a short promoter fragment of the potato gst1 gene. Molecular plant-microbe interactions: MPMI 9: 68-73 Xu C, Pasco DS (1998) Suppression of CYP1A1 transcription by H2O2 is mediated by xenobiotic-response element. Archives of biochemistry and biophysics 356: 142-150 Zheng H, Lin S, Zhang Q, Lei Y, Hou L, Zhang Z (2010) Functional identification and regulation of the PtDrl02 gene promoter from triploid white poplar. Plant Cell Rep 29: 449–460.