20070609093206805_2
advertisement

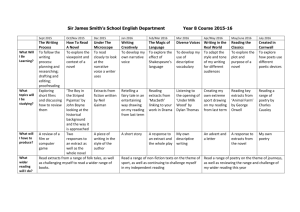
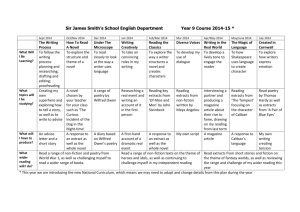
EFFICIENCY OF Ocimum sanctum.L EXTRACTS FOR ANTIMICROBIAL ACTIVITY AGAINST CERTAIN PATHOGENS Melvinjoe, M1, Jayachitra. J1, Vijayapriya. M1 and Pandeeswari, N1 1. Department of Microbiology, Faculty of Agriculture, Annamalai University ABSTRACT The in vitro antimicrobial activity of Ocimum sanctum leaf, root and inflorescence extracts were studied against selected pathogenic bacteria and fungi, following broth dilution assay. Leaves, root and inflorescence extracts were prepared using absolute alcohol, benzene, chloroform, methanol, petroleum ether, hot and cold water. Among all the solvents used the leaf extracts prepared using ethanol was found to be more effective against all the test microorganisms, which recorded minimum inhibitory concentration (MIC) ranged between 1.0 to 4.0 mg/ml. Ethanol extracts showed MIC of 1.0-4.0 mg/ml for bacteria and 2.0-4.0 mg/ml, for fungi. Extracts of benzene and petroleum ether were ineffective in inhibiting the bacterial and fungal growth or showed poor inhibition. The chloroform extract also showed a moderate antimicrobial activity of 4.0-8.0 mg/ml against the bacterial pathogens and 4.0-6.0 mg/ml against the fungal pathogens. The antimicrobial activity of Ocimum sanctum is discussed in the present paper. Keywords : Ocimum sanctum, minimum inhibitory concentration antimicrobial activity, extracts and secondary metabolites. INTRODUCTION Ayurvedic medicinal plants are rich in secondary metabolites which are potential source of drugs for approximately 25 per cent of prescribed medicines (Farnsworth and Bunyapraphatsara, 1992). The plants known as medicinal plants are rich in the secondary metabolites, which include alkaloids, glycosides, steroids and relative active metabolites which are used as drugs in pharmaceutical industry (Sobli and Pushphpangadan, 1982). The secondary metabolites are accumulated by plants in their leaves, roots and other organs. Despite the fact their biosynthetic origin and role in the plant are poorly understood, secondary metabolites are of considerable interest because of their potential industrial, pharmacological and medicinal value (Farnsworth and Bunyapraphatsara, 1992) Ocimum sanctum L. (Tulsi) or basil, queen of herbs has been used in Indian sub continent for thousand of years as a house hold remedy. The basil leaves are of high antimicrobial activity. The main components of leaves are tannins (4.6 per cent) and essential oils (2 per cent) such as flavonoids and phenolic acid. The amounts of the primary constituents of the essential oil vary according to the geographical distribution and variety of the source plant material such as Eugenol (upto 62 per cent), methyl eugenol upto 85 per cent) and and caryophyllene (upto 42 per cent) (Norr and Wagne 1992). The antimicrobial activity of Ocimum sanctum of Laminaceae family was well documented in the literature. The principle aim of the present work was to study antimicrobial activity of leaf, root and inflorescence. Extracts were prepared in different solvents like absolute alcohol, benzene, chloroform, methanol, petroleum ether, cold and hot water against human pathogenic bacteria including Bacillus cereus, Escherichia coli, Klebsiella pneumonia, Staphylococcus aureus, Vibrio parahaemolyticus and Salmonella typhi and the pathogenic fungi including the yeast like Candida albicans, Blastomyces dermatidis, Histoplasma capsulatum, Sporothrix schenckii, Cryptococcus neoformis. MATERIALS AND METHODS The first young plants of Ocimum sanctum were collected randomly during the month of January from the outskirts of Kanyakumari district, Tamilnadu, India. The plant species were identified by using the standard morphological characteristic features (key) according to the flora of Madras Presidency. Preparation of Extracts Fresh plant material (i.e. leaf, root and inflorescence) were washed and surface sterilized with 1 per cent ethanol and dried in hot air oven at about 35-40C and then powdered using a mechanical grinder, sieved through a sieve and stored in individual air tight bottles. 25 g of leaf, stem, root and inflorescence powder were extracted with 150 ml of solvent absolute alcohol, benzene, chloroform, methanol and petroleum ether for 25 hrs by using Soxhlet apparatus. The extracts were dried in a flash evaporator for 30 min and the left over powder was considered 100%. The hot and cold water extracts were prepared by maceration using a mortar and pestle and then filtering the extract by using Whatmann No.1 filter paper. Different concentrations were prepared by redisolving the extract powder in the same solvent which was used for the extraction. Test microorganisms Selected pathogenic bacteria Bacillus cereus MTCC 430, Klebsiella pneumoniae MTCC 2405, Staphylococcus aureus MTCC 3160, Vibrio parahaemolyticus MTCC 451, Salmonella typhi MTCC 734, Psuedomonas aerogenosa MTCC 4306 and fungi, the yeast like Candida albicans MTCC 183, Blastomyces dermatidis ATCC 26199, Histoplasma capsulatum ATCC 90723, Sporothrix schenckii ATCC 90723 and Cryptococcus neoformis ATCC 2517 were obtained from Institute of Microbial Technology India and American type cultural collection centre, U.S.A. respectively. All test bacterial species were maintained on nutrient agar media. The 36 hrs old bacterial culture were inoculated in to nutrient broth and incubated at 35 2C on a rotary shaker (Labortechnik, Germany) at 100 rpm. After 36 hrs incubation the bacterial suspension were centrifuged at 10000 xg for 15 min. The pellet was resuspended in sterile distilled water and the concentration was adjusted to 1108 Cfu/ml by reading the OD to 0.4 at 610 nm using UV-Visible spectrophotometer (Hitachi-U2000, Japan) and used for further studies. Fungal colonies were harvested from 9-10 day old cultures which were maintained on sabourauds agar. The spores were suspended in sterile distilled water and the spore suspensions were adjusted to 1108 spores/ml. Antimicrobial assay Antimicrobial assay were performed in 96 well sterile flat bottom microtiter plates based on broth microdilution assay which is an automated colorimetric method, uses the absorbance (optical density) of cultures. In a microtitre plate each well of microtitre plates were filled with 200l of nutrient broth 1l of test organism and 15l plant extract. The microtiter plates were incubated at 35 2C for 24hr. After the incubation period the plates were read at 465 nm using ELISA reader (ELX 800 MS, Biotek Instruments Inc. USA) MIC, determined as the lowest concentration of plant extract inhibiting the growth of the organism were determined based on the readings. RESULTS AND DISCUSSION The result of the antimicrobial screening tests of leafs, roots and inflorescence extracts of O. sanctum in different solvents were tested against human pathogenic microorganisms using microdilution technique are depicted in Tables 1 and 2. The extracts are found to be more effective against bacteria rather than fungi both benzene and petroleum ether extracts were found to be ineffective or showed poor inhibition on bacterial and fungal growth. The microdilution assay gives MIC values ranging from 1.0-80.0 mg/ml for different microorganisms (Tables 1 and 2) the lower MIC values were obtained for leaf extracts obtained with ethanol which showed to 1.0 mg/ml for B. cereus, S. aureus followed by chloroform extract which showed a least MIC of 1.0 against S. aureus. The cold and hot a water extract also showed some, activity against some of the pathogens. The results of the antimicrobial activity of the root extract showed a moderate inhibitory effect against most of the pathogens which varied between 2.0 mg/ml to 80.0 mg/ml against the pathogens. The low MIC values were observed in the chloroform extracts i.e. 2.0 for C. albicans and H. capsulatum the inflorescence sample showed the least inhibitory effect against all the organisms tested which showed a MIC ranging from 8.0 mg/ml to 80.0 mg/ml, in which the alcoholic extract showed the least MIC of 6.0 for B. cereus and S. aureus. The antimicrobial activity of O. sanctum extracts varied with plant parts and the solvents used for extraction. The yields of the cold and hot water extracts were relatively low. Maceration has generally reported to give low yield of plant extracts as compared to Soxhlet extraction (Machado et al., 1999). The higher antimicrobial property exhibited by the ethanol, benzene and chloroform extracts, over water extracts show that higher proportion of plant components were water insoluble. It has been found that most of the extracts contained the metabolites, though not in the same proportions, and also the active principle were more soluble in analytical ethanol as compared to the other solvents. The disparity in antimicrobial activity between the cold and hot water extracts over other solvents is due to the fact that solvent extracts have reported to contain higher amounts of plant constituents (Okete et al., 2001, Okoli et al., 2002). But in some cases the cold water extract exhibited better antimicrobial activity as compared to the hot water extract it may be due to the fact that heat inactivation of some of the secondary metabolites in the hot water extract. The antibacterial activity of the extracts against different pathogenic organisms is due to the presence of tannins, essential oils, flavonoids, alkaloids and eugenol in varying proportions of O. sanctum (Lakakso et al., 1990). The lower inhibitory effect of the root and inflorescence samples may be attributed to the fact that the presence of low concentrations of antimicrobial components as compared to the leaf extracts (Leven et al., 1999). Phytochemicals exert their antimicrobial activity through different mechanisms, tannins for example act by iron deprivation, hydrogen bounding or non-specific interactions with vital proteins such as enzymes (Scalbert, 1991). The alkaloids act as a DNA intercalator and an inhibitor of DNA synthesis through topoisomerase inhibition (Passonen et al., 2002, Guittat et al., 2003). The lower inhibtiory effect of the extracts against the fungal pathogens may be attributed to the fact that they are resistant to the most commonly used antibiotics and also due to the fact that they have rigid chitin in cell wall. It has been found that the gram positive bacteria were more susceptible to the extracts as they have only on outer peplidoglycan layer, which is not an effective barrier (Scherrer et al., 1971). The gram negative bacteria have an outer phospholipidic membrane that makes the cell wall impermeable to lipophilic solutes, to while the porins constitute a selective barrier to hydrophilic solutes with an exclusion limit of about 600 Da (Nikaido and Varara, 1985). Many results confirmed these observations, thus some plant extracts were found to be more active against gram-positive bacteria than gram negative ones (Kelmanson et al., 2000, Mesika and Atolsyane 2002). In conclusion ehtanolic and chloroform extracts of Ocimum sanctum showed excellent antimicrobial activity against all the test microorganisms. Further work is needed to isolate the active principle from the plant extracts to carry out pharmaceutical studies. REFERENCES [1] Farnsworth, N.R.; Bunyapraphatsara, N.: Thai medicinal plants. Medical Plant Information Center, Bangkok, 1992; 180-182. [2] Sobli, S.N.; Pushphpangadan, P.: Studies in the genus Ocimum: Cytogenetics, breeding and production of new strains of economic importance. In: Atal, C.K., Kapur, B.M. (eds) cultivation and utilization of aromatic plants. Regional Laboratory Council of Scientific and Industrial Research, JammuTawi, India, 1982, 457-472 [3] Norr, H.; Wagne, H.: New constituents from O. sanctum. Planta Med. 58; 574, 1992. [4] Machado, M.G.; de Vasconcelos Silva, P.J.; Mutos, A.A; Craveiro and Alencer, J.W.: Volatile constituents from leaves and inflorescence oil of Ocimum tenuflorum L. F (Syn. O. sanctum) grown in Northeastern Brazil. J. Essent. Oil Res. 11; 324-326, 1999. [5] Okeke, M.I.; Inoeglu, C.U.; Eze, E.N.; Okoli, A.S.; Esimone, C.O.: Evaluation of extracts of the root of Landolphia occurrence for antibacterial activity. J. Ethnopharmacol., 78; 119-127, 2001. [6] Okoli, A.S.; Okeke, M.I.; Iroeghu, C.U.; Ebo, P.U.: Antibacterial activity of Harungana madagascariensis leaf extracts. Phytother., 16; 174-179, 2002. [7] Laakso, J.; Seppsnen-Laakso, T.; Herrmann-Wolf, B.; Kuhosel, N.; Knobloch, K.: Constituents of the essential oil from the holy basil of Tulsi plant, O. sanctum. Planta. Med., 56; 527, 1990. [8] Leven, M.D.; Vanden-Berghe, D.A.; Marten, T.; Vilientrick, A.; Lomweas, E.C.: Screening higher plants for biological activity. Planta Med., 36; 311-312, 1999. [9] Scalbert. Antimicrobial properties of tannins. Phytochemistry, 30; 3875-3883, 1991. [10] Dassonne Ville, L.; Lansiaux, A.; Wattelet, A.; Wattez, N.; Mathieu, C.; Vanmiert, S.; Pieters, L.; Bailly, C.: Cytotoxicity and cycle effect of the plant alkaloids cryptolepime and leocryptole pine relation drug-induced apotosis. Eur. J. Pharmacol., 409; 9-18, 2002. [11] Guittat, L.; Alberti, P.; Rosu, F.; Vanmiert, S.; Thetiot, E.; Pieters, L.; Gabelia, V.; De Pauw, E.; Ottaviani, A.; Roju, J.F.; Meigny, J.L.: Interaction of cryptocepine and neocryptolepine with unusual DNA structures. Biochem., 85; 535-541, 2003. [12] Scherrer, R.; Lerlard, P.: Molecular sieving by the Bacillus megatherium cell well and protoplast. J. Bacteriol., 107; 718-735, 1971. [13] Nikaido, H.; Varara, M.: Molecular basis of bacterial outer membrane permeability. Microbial Rev., 1; 1-32, 1985 [14] Kelmanson, J.E.; Jagur, A.K.; Van Staden, J.: Zulu medicinal plants with antibacterial activity. J. Ethnophermacol, 69; 241-246, 2000. [15] Mesika, P.L.; Afolsyane, A.J.: Antimicrobial activity of some plants used for the treatment of livestock diseases J. Ethnophermacol, 83; 129-134, 2002. in eastern cape. South Afr. Table 1. Minimum inhibitory concentration (MIC) of Ocimum sanctum for antibacterial activity MIC (mg/ml) Source Solvents Ocimum sanctum Alcohol Benzene Chloroform Leaf Methane Petroleum ether Cool water Hot water Alcohol Benzene Chloroform Root Methane Petroleum ether Cool water Hot water Alcohol Benzene Chloroform Inflorescence Methane Petroleum ether Cool water Hot water Chromamphenical B. cereus K. pneumoniae S. aureus V. parahaemolyticus S. typhi P. aeroginosa 1.0 1.0 * 30.0 4.0 6.0 80.0 6.0 8.0 * 4.0 2.0 6.0 * * * 8.0 10.0 20.0 20.0 6.0 1.0 1.0 1.0 4.0 6.0 * * 6.0 6.0 2.0 2.0 8.0 * 10.0 20.0 8.0 30.0 4.0 2.0 6.0 1.0 10.0 30.0 4.0 30.0 80.0 4.0 6.0 4.0 8.0 20.0 80.0 80.0 * 4.0 Values are a mean 3 determinations - = no value; * Shows poor inhibition of bacterial growth Table 2. Minimum inhibitory concentration (MIC) of Ocimum sanctum for antifungal activity MIC (mg/ml) Source Solvents Ocimum sanctum Alcohol Benzene Chloroform Leaf Methane Petroleum ether Cool water Hot water Alcohol Benzene Chloroform Root Methane Petroleum ether Cool water Hot water Alcohol Benzene Chloroform Inflorescence Methane Petroleum ether Cool water Hot water Fuconazole C. albicans B. dermatidis H. capsulatum S. schenckii C. neoformis 2.0 * 4.0 * 10.0 20.0 6.0 2.0 4.0 * 20.0 80.0 6.0 10.0 * * 4.0 4.0 6.0 * * 8.0 4.0 10.0 10.0 20.0 6.0 2.0 2.0 * 4.0 2.0 10.0 8.0 10.0 4.0 2.0 4.0 * 4.0 4.0 6.0 8.0 30.0 2.0 4.0 10.0 4.0 * * 8.0 20.0 6.0 20.0 * * * * 6.0 Values are a mean 3 determinations - = no value; * Shows poor inhibition of bacterial