Mineral resources
advertisement

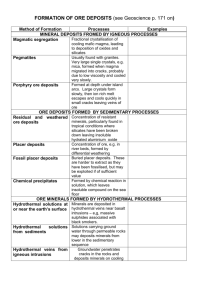
Mineral resources Metalliferous ores Most of the elements are metals. The properties of metals make them particularly useful to humans. Due to their reactivities, most metals combine with other elements to form compounds but some metals such as gold, silver and sometimes copper are so unreactive they do not form compounds, they are found native (uncombined). Metals can only be extracted from an ore if it is economic to do so, in other words, the cost of extraction and processing is less than the selling price. The nature of mineral deposits All mineral reserves are finite resources. Most metals are present in such small quantities that it is necessary for the metal to be concentrated for any ore to be economic, fortunately there are several natural processes which serve to concentrate the metal in an ore deposit. Formation of mineral deposits Mineral deposits are formed either by internal processes (associated with igneous activity or metamorphism) or by surface processes (weathering, erosion, transport etc.) Magmatic segregation This is an igneous process. Certain ore minerals such as magnetite, chromite and ilmenite form from basic (and ultrabasic) magma. They form early in the crystallisation sequence and because they are denser than the surrounding magma, they sink to the bottom of the magma chamber. These crystals accumulate in layers called cumulates. One famous example of the type of ore body is the Bushveld intrusion in South Africa. Diagram showing magmatic segregation. Another way in which a magma may produce a ore depsoits involves dense liquids within the magma (containing iron, nickel and copper compounds) sinking and crystallising at the bottom of the magma chamber. Hydrothermal veins During the late stages of magma intrusion, hot (600 C) metal-rich fluids called brines circulate out of the magma and into the surrounding rocks. These watery solutions pass through cracks in the surrounding country rock, cooling as it does so. The lower temperature causes certain minerals to be precipitated along the walls of the crack. This is called a mineral vein. A typical hydrothermal vein. Cornish tin deposits are hydrothermal veins. Cassiterite crystallises at high temperatures and pressures, so it tends to be found close to the magma chamber. Chalcopyrite, sphalerite, iron pyrites, galena and haematite occur further away. Ore minerals are found in association with worthless minerals (called gangue) including quartz, calcite and fluorite. Sometimes the ore becomes spread through tiny fractures, these are called disseminated deposits, the porphyry copper deposits in America are an example. Contact metasomatism This is a metamorphic process. Hot watery liquids produced by magma contain chloride and fluoride ions. This liquid is acidic and it can affect surrounding rocks particularly limestones. The reaction produces new minerals, copper, lead and zinc sulphides and iron oxides in particular. Pegmatites In a granitic magma, the last liquid to crystallise is rich in rare elements and water as well as silicates. This liquid cools very slowly to form veins containing very large crystals of many different minerals such as feldspar and mica as well as semi-precious gems and minerals containing rare elements such as lithium, caesium and uranium. Oceanic ridges Hydrothermal deposits are found at the oceanic ridges, but the water does not come from a magma, instead it is seawater which has seeped through (percolated) the newly-formed oceanic crust. The water becomes heated and it is then able to dissolve minerals from the basalt. The heat causes a circulation of the water, back to the ocean. When the hot water emerges into the sea, it quickly cools and the minerals it carries in solution are precipitated in chimneys called black smokers. Most of the minerals formed in these black smokers are iron and copper sulphides with some other metals. Formation of a black smoker Secondary enrichment This process converts a dispersed ore body into a concentrated one. It is caused by chemical reactions between minerals in different oxidising conditions. Near the surface a chemical reaction occurs which converts insoluble sulphides to soluble sulphates. Chalcopyrite + Water + Oxygen Copper sulphate + Iron hydroxide The sulphates are carried down by percolating water. Some of the copper is deposited as copper carbonate and oxide in the oxidising environment just above the water table. Below the water table copper ions react with more chalcopyrite to form chalcocite. Copper ions + Chalcopyrite Chalcocite + Iron sulphide The residual deposits of iron hydroxide at the surface tends to be a rusty brown colour. This is sometimes referred to as an “iron hat” or gossan and it is a good indicator of the presence of an ore deposit below the surface. Residual deposits Weathering processes (particularly in hot, wet climates) produce thick layers of laterite, a type of soil. Certain insoluble minerals become concentrated in this layer, while the soluble weathering products are washed away. Bauxite forms in this way: Potassium feldspar + Acidic rainwater Kaolinite + K+ ions + Silica Kaolininte + Water Bauxite + Silica Chemical precipitation Water at the surface of the Earth contains many different ions. Under certain conditions, these ions may be precipitated. During the Precambrian, the atmosphere was mainly composed of carbon dioxide. When this is dissolved in water, it forms an acidic solution. Under these conditions, the water contained iron in the 2+ form. When this water mixed with oxygenated water (caused by photosynthetic bacteria), the iron became oxidised to the 3+ ion, precipitating as iron (III) oxide. This type of deposit is called a banded iron formation. These deposits mainly contains haematite mixed with other minerals. They are low grade ores and they have to be processed before being used in the blast furnace. Placer deposits Weathering and erosion of an ore body may liberate fragments of economic minerals. These fragments are transported which sorts the fragments according to their densities. Dense fragments are deposited quickly and may accumulate in cracks and depressions on the bed or on the inside of a meander. The placer deposit may be more concentrated than the original ore body. The minerals concentrated as placers must be tough and resistant to both mechanical disintegration and chemical breakdown. Gold is the most famous example of this type of ore deposit but placers are also important sources of tin (cassiterite), platinum and diamonds. Placers can be a good indicator of the locations of the parent ore body, by following the stream back it may be possible to locate the source, this is how the kimberlite pipes of South Africa were discovered.