Supplemental material
advertisement
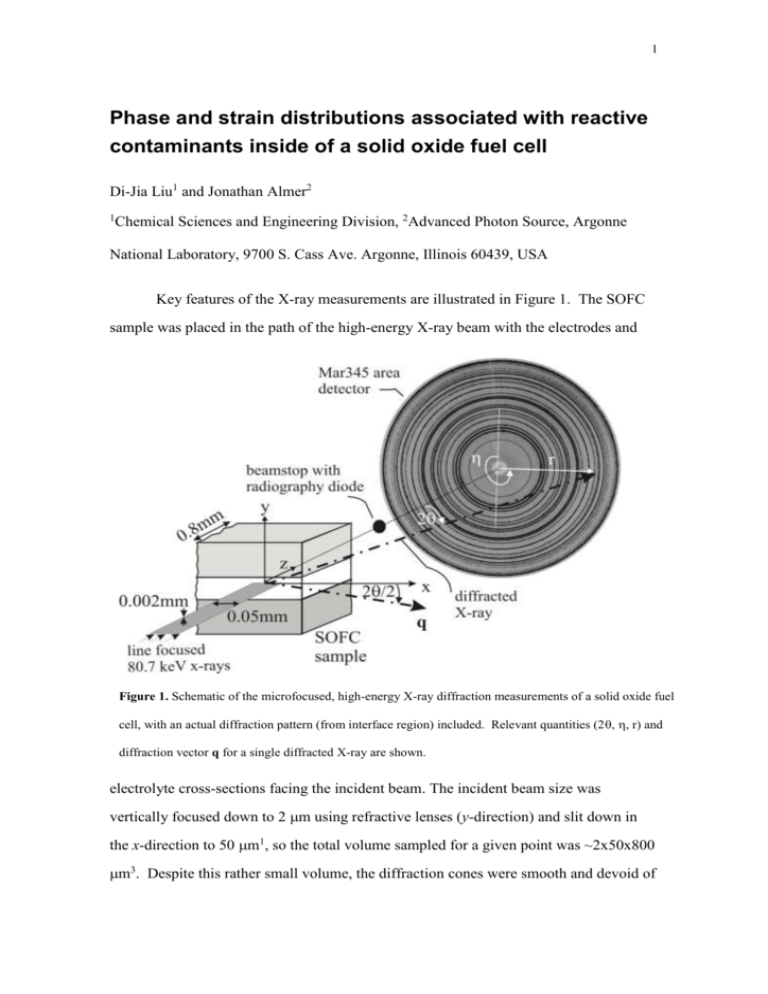
1 Phase and strain distributions associated with reactive contaminants inside of a solid oxide fuel cell Di-Jia Liu1 and Jonathan Almer2 1 Chemical Sciences and Engineering Division, 2Advanced Photon Source, Argonne National Laboratory, 9700 S. Cass Ave. Argonne, Illinois 60439, USA Key features of the X-ray measurements are illustrated in Figure 1. The SOFC sample was placed in the path of the high-energy X-ray beam with the electrodes and Figure 1. Schematic of the microfocused, high-energy X-ray diffraction measurements of a solid oxide fuel cell, with an actual diffraction pattern (from interface region) included. Relevant quantities (2, , r) and diffraction vector q for a single diffracted X-ray are shown. electrolyte cross-sections facing the incident beam. The incident beam size was vertically focused down to 2 m using refractive lenses (y-direction) and slit down in the x-direction to 50 m1, so the total volume sampled for a given point was ~2x50x800 m3. Despite this rather small volume, the diffraction cones were smooth and devoid of 2 texture, as can be seen in Figure 1, which is a consequence of the fine grain sizes and processing of the SOFC layers. The SOFC was mounted on stages to allow translation in x, y and z, as well as tilting relative to the fixed X-ray beam. The x and y-translations enabled two dimensional mapping of the sample while the z translation ensured the optimal focus and same sample-detector distance as the CeO2 calibrant, see below. The X-ray detection and data collection are reported in the main text. Due to the low Bragg angles (1°< <4°) associated with the 80.7 keV X-rays and transmission geometry employed, the 2-d diffraction patterns (see Fig.1) contain information from detector orientations () which are nearly parallel (offset by ) to the SOFC in-plane (x, = ±/2) and vertical (y, = n) directions. Measured radii were converted to absolute d-spacings by measuring a NIST-calibrated CeO2 powder under the same conditions2. By binning intensity data into a finite set of orientations (N= 72, each bin representing 5°) and carefully measuring the radius/d-spacing of a given reflection versus azimuthal bin (dhkl())a, the deviatoric (directional) strain is computed as: hkl = (dhkl,x-dhkl,y)/dhkl,ave (1) where dhkl,y (dhkl,x) are d-spacings along the vertical (in-plane) directions, respectively, as derived from a least-squares fit to dhkl( over all azimuth bins and with geometry accounted for, and dhkl,ave is the orientation-averaged d-spacing. The orientationaveraged intensities Iave (N= 1), which are insensitive to deviatoric strain, were used for Reitveld refinements in GSAS3 and analyzed over the range 1 ≤ d ≤ 4 Å. Selected parameters used for this analysis are given in Table 1. For each of the phases present (i a Symbols in bold denote vector quantities which vary in both x and y directions of the sample. 3 = 1-5)b, thermal parameters, intra-phase compositions and atomic positions were fixed while lattice parameters and weight fractions wi were refined. Table I. Selected data for GSAS analysis Phase (nominal Space Group composition) Nominal lattice Density parameters (Å) (g/cm3) La1-XSrXMnO3 (x=0.8) R3-C (167) a=5.462; c=13.436 6.56 Zr1-XYXO2 (x=0.84) FM3-M (225) 5.136 6.01 Ni FM3-M (225) 3.524 8.90 Cr2O3 R3-c (167) a=4.959; c=13.594 5.21 Mn(Mn0.193Cr1.808)O4 Fd3-m (227) 8.447 4.91 The observed strains induced by the deposition of chromia can be attributed primarily to thermal origins. As can be seen in the Table II below, Cr2O3 has a lower thermal expansion coefficient (TEC) than all other (parent) phases in the SOFC. In addition, the heterogeneous mass transfer of Cr from the interconnect into the cathode at elevated temperature reduces porosity in Cr2O3-rich regions (data to be published) which leads to a region of effectively higher constraint than the more porous surrounding regions. These combined effects lead to a lower effective TEC in the chromia accumulated zone than that in the surrounding matrix, leading to compressive b Phase detectability was approximately 0.1wt%; in cases where less than 0.1wt% of a given phase was present in a given run, Rietveld analysis was re-run without that phase present. 4 residual strains in this region upon cooling. The thermal expansion mismatch, , needed to form such in-plane compressive strains x can be estimated as =x/T, where x~0.6hklc. Using T=800ºC and hkl≈-1.5x10-3 from the experiment, gives =-1x10-6 C-1, which is on the same order of observed TEC differences. On the other hand, growth-induced strains in Cr2O3 could also play a role in the measured residual strains. While little extant information on Cr2O3-induced growth strains is available, systematic investigations by Tolpygo and Clarke4 on Al2O3 scale growth can be used as a reference. They showed that while growth stresses were appreciable, and could be either tensile or compressive pending scale thickness, the dominant source (by a factor of ~3) of residual strains was thermal-induced mismatch. In-situ observations, as a function of temperature, on Cr-poisoned films would be necessary to further resolve the relative contributions. Plastic deformation would not be expected in any of these ceramic-based materials such as SOFC, and indeed we see no evidence of relative peak broadening within the chromia-rich regions, in any of the phases, which would be expected in the presence of extensive plastic deformation. In the presence of irreversible processes, namely plastic deformation and/or creep, thermal cycling of Cr-poisoned systems would be expected to further degrade the SOFC, perhaps even sooner than the single-thermal-cycle investigated here. While c Assuming an equi-biaxial stress state, common in layered systems, the relationship between the in-plane x and measured deviatoric strains = (x-y) is dependent on the Poisson ratio as x/=(1-)/(1+), or 0.6 using a typical value for of 0.25. 5 creep behaviour was not studied here, it would be another natural extension of present work via in-situ studies. Table II. Thermal expansion coefficients of SOFC materials between room temperature and 1000ºC. Thermal Expansion Reference Phase/Component Coefficient (x10-6/ºC) Cathode: La1-xSrxMnO3 (x~0.8) Electrolyte: Zr1-xYxO2- (x~0.84) 12.4 10.5-11.0 5 5, 6 Anode: Ni/YSZ cermet (30 vol% Ni) 12.3 5 Cr2O3 7.7 7 References 1. S. D. Shastri, J. Almer, C. Ribbing and B. Cederström, J. Synchrotron Rad. 14, 204, (2007). 2. J. Almer, U. Lienert, R. L. Peng, C. Schlauer, and M. Oden, J. App. Phys., 94 (1), 697, (2003). 3. A. C. Larson, and R. B. Von-Dreele, General Structure Analysis System (GSAS), Los Alamos National Laboratory Report LAUR 86-748, 2000. 4. V. Tolpygo and D.R. Clarke, Oxidation of Metals 49 (1/2), 187, (1998). 5. N.Q. Minh, J. Am. Ceram. Soc. 76, 563, (1993). 6. F. Tietz, Ionics 5, 129, (1999). 6 7. Thermophysical Properties of Matter, Y.S. Touloukian, series ed (Plenum Press, New York, 1977).