Prevention and Treatment of RSV
advertisement

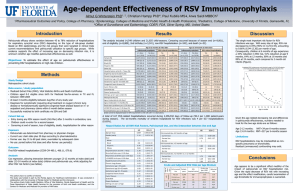
PREVENTION AND TREATMENT OF RSV INFECTIONS. NEW DRUGS ON THE PIPELINE Octavio Ramilo, MD Division of Infectious Diseases and Center for Vaccines and Immunity Nationwide Children’s Hospital and The Ohio State University College of Medicine Despite the considerable impact that respiratory syncytial virus (RSV) infections have in the health of children and adults, the development of therapeutic interventions has been remarkably slow. The initial efforts to develop an effective vaccine in the 1960s resulted in significant failure and delayed the progress for decades. Ribavirin was the first antiviral agent licensed for treatment of RSV infections in the 1980s, however, its use has remained limited for different reasons. It was only in the 1980s when advances in the characterisation of neutralising antibodies against RSV led to the development and clinical application of a polyclonal immunoglobulin preparation with high titres of neutralising antibodies against RSV (RSV IVIG). This preparation demonstrated efficacy in reducing hospitalisations due to severe RSV infections in high-risk children and established the proof of principle of the value of anti-RSV antibodies. The next step was the development of neutralising monoclonal antibodies. Among several antibodies evaluated in clinical studies, only palivizumab demonstrated clinical efficacy and was licensed in 1998 by the FDA, and subsequently by the different world drug regulatory agencies for prevention of severe RSV infections in high-risk children. Motavizumab (MEDI-524) is a novel recombinant humanised IgG1 mAb derived from palivizumab with enhanced anti-RSV neutralising activity. A phase III clinical study comparing motavizumab versus palivizumab for prevention of RSV infection in high-risk children has been recently completed. Motavizumab was non inferior to palivizumab and demonstrated a 26% reduction in RSV hospitalisations compared with palivizumab. In addition, motavizumab demonstrated superior efficacy compared to palivizumab with a 50% reduction in RSV lower respiratory infections requiring medical outpatient treatment. In recent years, in addition to anti-RSV antibodies, a number of alternative strategies against RSV are being developed. The majority of the new anti-RSV therapies involve designing small molecules with antiviral activity, and there is a renewed interest in developing vaccines. However, the most innovative approach is the use of siRNAs (small inhibitory RNAs) with specific anti-RSV activity, which will represent a novel therapeutic concept for treatment of human disease. These molecules have demonstrated excellent antiviral activity in animal models of RSV infection, and initial studies in humans are already underway.