Supplementary Table 1. Inclusion criteria Inclusion criteria 1) Men
advertisement

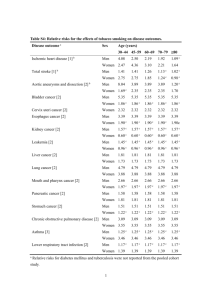
Supplementary Table 1. Inclusion criteria Inclusion criteria 1) Men and women who at time of informed consent were 40 years or older 2) Diagnosed with COPD by respiratory specialist doctor 3) Patients not receiving regular COPD treatment 4) Outpatient smoking history of 10 pack years or more 5) Percentage of the predicted value of FEV1 of 80% or less* 6) FEV1/FVC after bronchodilator inhalation of less than 70% 7) Result of pre examination by the doctor indicated the patient was eligible 8) Patients who signed informed consent forms FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease. *Prediction formula of FEV1: (male): Predicted value of FEV1 (L) = 0.036 × height (cm) − 0.028 × age − 1.178; (female): Predicted value of FEV1 (L) = 0.022 × height (cm) − 0.022 × age − 0.005. Supplementary Table 2. Exclusion criteria Exclusion criteria 1) Persons with a history of hypersensitivity to any ingredient in each standard therapy 2) Patients with a disease contraindicated in the package insert of each standard therapy 3) Abnormal laboratory values or a clinical problem was observed 4) Alcohol or drug abusers 5) Patients unable to undergo non-smoking guidance 6) Patients with bronchial asthma, cystic fibrosis, bronchiectasis, interstitial lung disease, pulmonary thrombosis and pulmonary embolism 7) Persons with a history of thoracotomy with lung resection 8) Those with planned lung volume reduction surgery or lung transplantation 9) Those who could not perform pulmonary function tests 10) Persons who had regularly used inhaled steroids or long-acting bronchodilators before the start of the survey 11) Those who were taking the study drug, or who took part in the clinical trial or other clinical trials within 2 months before obtaining informed consent 12) Persons pregnant or suspected of being pregnant, or who were breastfeeding 13) Others determined to be inappropriate by the examining doctor Supplementary Table 3. Drugs administered in this study Product name General name Route of administration Pharmaceutical company Pharmaceutical company country Long-acting bronchodilator agents Serevent Salmeterol xinafoate Inhalation GlaxoSmithKline K.K. Hokunalin Tape Tulobuterol Transdermal Abbott Japan Co., Ltd. UK (GlaxoSmithKline plc) USA (Abbott Laboratories) Theophyllines Uniphyl LA Theophylline Oral Otsuka Pharmaceutical Co., Ltd. Japan Inhalation GlaxoSmithKline K.K UK (GlaxoSmithKline plc) Japan Short-acting bronchodilator agents Sultanol Salbutamol sulfate Meptin Procaterol hydrochloride Inhalation Clickhaler hydrate Inhaled corticosteroid/long-acting bronchodilator agents Advair Fluticasone Inhalation propionate/Salmeterol xinafoate Symbicort Budesonide/Formoterol Inhalation fumarate hydrate Mucolytic agents Mucodyne L-carbocisteine Oral Methista L-carbocisteine Oral Otsuka Pharmaceutical Co., Ltd. GlaxoSmithKline K.K UK (GlaxoSmithKline plc) AstraZeneca K.K UK (AstraZeneca plc) Kyorin Pharmaceutical Co., Ltd. Towa Pharmaceutical Co., Ltd. Japan Japan Short-acting muscarinic antagonist Tersigan Oxitropium bromide Inhalation Boehringer Ingelheim Japan, Inc. Germany (Boehringer Ingelheim GmbH) Smoking cessation agent Champix Varenicline tartrate Oral Pfizer Japan Inc. USA (Pfizer Inc.) Supplementary Table 4. Analysis of the ITT group that reached the pharmacologic maintenance phase. Stage of smoking behavior in past smokers Visit 1 at baseline 0 0 1 12 Visit 2 at Week 8 0 0 1 11 Visit 3 at Week 16 0 0 3 6 Visit 4 at Week 24 0 0 1 3 Visit 5 at Week 36 0 0 3 0 Visit 6 at Week 48 0 1 1 3 Precontemplation (smoking) Contemplation (smoking) Preparation (smoking) Action (under smoking cessation) Maintenance (under smoking cessation) Unknown Stage of smoking behavior in current smokers 26 27 30 31 32 28 0 Visit 1 at baseline 2 2 6 0 0 Visit 2 at Week 8 2 1 2 5 0 Visit 3 at Week 16 1 2 2 3 4 Visit 4 at Week 24 0 1 0 4 4 Visit 5 at Week 36 0 0 0 2 6 Visit 6 at Week 48 0 1 1 2 Precontemplation (smoking) Contemplation (smoking) Preparation (smoking) Action (under smoking cessation) Maintenance (under smoking cessation) Unknown 0 0 2 3 5 4 0 0 0 2 3 2