Supplementary Information (doc 123K)
advertisement

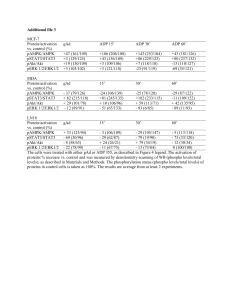
SUPPLEMENTARY INFORMATION Targeting ErbB-2 Nuclear Localization and Function Inhibits Breast Cancer Growth and Overcomes Trastuzumab Resistance Rosalía I. Cordo Russo, Wendy Béguelin, María Celeste Díaz Flaqué, Cecilia J. Proietti, Leandro Venturutti, Natalia Galigniana, Mercedes Tkach, Pablo Guzmán, Juan C. Roa, Neil A. O'Brien, Eduardo H. Charreau, Roxana Schillaci, and Patricia V. Elizalde SUPPLEMENTARY FIGURE LEGENDS Supplementary Figure S1. Effect of hErbB-2∆NLS on HRGβ1-induced ErbB-2 activation and cellular localization, and on HRGβ1-induced BC growth. (a) HRGβ1 induces ErbB-2 nuclear migration in BC cells. C4HD cells were treated as indicated and ErbB-2 (red) was localized by immunofluorescence and confocal microscopy. Nuclei were stained with DAPI (blue). (b) Effect of hErbB-2NLS on endogenous ErbB-2 nuclear migration. C4HD cells displaying membrane ErbB-2 overexpression were transfected with the hErbB-2NLS mutant and treated with HRGβ1. Green fluorescent protein (GFP) from hErbB-2NLS expression vector was visualized by direct fluorescence imaging (green), and mouse ErbB-2 (red) was localized using an antibody that specifically recognizes the mouse protein. Solid arrows: cells transfected with hErbB-2NLS, dashed arrows: wild-type cells that did not uptake the hErbB-2NLS mutant. See Supplementary Materials and Methods for specifications of antibodies used in a and b. (c) The hErbB-2NLS mutant induces ErbB-2 and Stat3 phosphorylation in response to HRGβ1. Cells were transfected with siRNAs targeting mouse ErbB-2 or with control siRNAs and cotransfected with hErbB2WT or hErbB-2NLS plasmids, and then treated with HRGβ1. Western blots (WB) were performed with pTyr ErbB-2 and Stat3 antibodies and then membranes were reprobed with the respective total protein antibody. Membranes were also probed with an Actin antibody. Experiments shown in a to c were repeated three times with similar results. (d) Nuclear ErbB-2 drives HRGβ1-induced in vitro proliferation. C4HD cells were transfected with ErbB-2 siRNA and cotransfected with hErbB-2WT or hErbB2NLS or were transfected with control siRNA and cotransfected with hErbB-2NLS before HRGβ1 stimulation for 48 h and were then stained with propidium iodide (PI) and analyzed for cell cycle distribution by flow cytometry. The experiments shown are representative of a total of three. (e) Intratumoral expression of hErbB-2∆NLS at the end of the experiment. Since the experimental strategy relied on transient transfection of C4HD cells with the GFP-tagged hErbB-2∆NLS, its content was analyzed by flow cytometry. Left panel: shown is a representative sample of each tumor type. Right panel: percentage of GFP-positive cells in tumors from each group. Values are shown as mean ± s.d. (n=3). For b vs. a: P<0.001. Related to Figure 1. Supplementary Figure S2. (a) Quantitative analysis of ErbB-2 subcellular localization in confocal images of JIMT-1 cells from Figure 3a. Fluorescence intensities of ErbB-2 localized at the membrane (MErbB-2) and the nucleus (NErbB-2) were quantified in 50 cells from each group and are plotted as the percentage of MErbB-2 (left) or the percentage of NErbB-2 (right) (mean ± s.d.). (b) Cellular localization of ErbB-2 in Ttzm-resistant BC cells. Cells were treated as indicated and ErbB-2 (red) was localized by immunofluorescence and confocal microscopy. Boxed areas are shown in detail in the right inset. Nuclei were stained with DAPI (blue). (c) Stat3 levels in BT-474 and BT-474HR cells. Left panel: Stat3 expression was analyzed by WB. Right panel: Stat3 (red) was localized by immunofluorescence and confocal microscopy. Nuclei were stained with DAPI (blue). Total Stat3 levels in confocal images were quantified and are plotted as the average (mean ± s.d.) fluorescence intensities (integrated intensity per unit area) in 30 cells. (d) Effect of hErbB-2NLS on ErbB-2 nuclear migration in Ttzm-resistant cell lines. Cells were transiently transfected with the hErbB2NLS mutant and treated with HRGβ1 or remained untreated. GFP from hErbB2NLS expression vector was visualized by direct fluorescence imaging (green), and total ErbB-2 (red) was localized by immunofluorescence. Solid arrows: cells transfected with hErbB-2NLS, dashed arrows: wild-type cells that did not uptake the hErbB-2NLS mutant. Nuclei were stained with DAPI (blue). See Supplementary Materials and Methods for specifications of antibodies used in panels b, c, and d. The experiments from which the data were obtained are representative of three independent experiments. Related to Figure 3. Supplementary Figure S3. (a) Inhibition of ErbB-2 nuclear localization abolishes HRGβ1-induced in vitro proliferation in T47D cells. Cells were transfected with the hErbB-2NLS or pcDNA3.1 vector and were then treated with HRGβ1 48 h or remained untreated. Incorporation of [3H]thymidine was used as a measure of DNA synthesis. Data are presented as mean ± s.d. For b vs. a, and c vs. b: P<0.001. (b) AG825 inhibition of HRGβ1-induced ErbB-2 phosphorylation. Cells were treated with HRGβ1 or were preincubated with AG825 for 90 min before HRGβ1 stimulation. WB were performed with phospho (p) ErbB-2 antibodies and filters were reprobed with total ErbB-2 antibody. (c) Ttzm fails to block ErbB-2/Stat3 colocalization in BT-474 and JIMT-1 cells. Cells were treated with Ttzm or pretreated with Ttzm before HRGβ1 stimulation. ErbB-2 (green) and Stat3 (red) were localized by immunofluorescence and confocal microscopy. Merged images show ErbB-2 and Stat3 nuclear colocalization in yellow. Boxed areas are shown in detail in the right inset. Nuclei were stained with DAPI (blue). Experiments in a to c were repeated three times with similar results. Related to Figure 4. Supplementary Figure S4. HRβ1 induces cyclin D1 protein expression via ErbB2 and Stat3 in T47D cells. (a) Cells were treated with HRGβ1 for the pointed times and cyclin D1 protein expression was analyzed by WB. (b) Cells were preincubated with Jak inhibitor I or with AG825 or transfected with Stat3 and control siRNAs and were then treated with HRGβ1 for 48 h. Cyclin D1 expression was studied by WB. Right panel: control of inhibition of Stat3 expression by siRNAs. Experiments in a and b were repeated five times with similar results. Related to Figure 5. Supplementary Figure S5: Nuclear Stat3 score in MErbB-2-overexpressing tumor samples. Nuclear Stat3 (NStat3) levels were evaluated by immunofluorescence and scored considering both the percentage of positive cells and staining intensity. A score of 0 represents faint or no staining in less than 10% of cells, 1+ weak nuclear staining in 10-25%, 2+ moderate staining in 26-50%, and 3+ strong staining in > 50% of cells. Score of 1+, 2+, and 3+ were considered positive for NStat3 presence. Shown are examples of TMAs showing 0 to 3+ NStat3 staining. Nuclei were stained with DAPI (blue). Related to Figure 7. SUPPLEMENTARY MATERIALS AND METHODS Antibodies The following antibodies were used for Western blots: phospho-Stat3 (Tyr705) (B-7), total Stat3 (C-20), ErbB-2 (C-18, raised against the C terminus), ErbB-2 (9G6, raised against the N terminus), ErbB-3 (C-17), and phosphotyrosine (PY99), all from Santa Cruz Biotechnology (Santa Cruz, CA, USA); phospho-ErbB-2 (Tyr1221/1222), phospho-ErbB-2 (Tyr877), phospho-Akt (Ser473), and Akt (C67E7), from Cell Signaling (Beverly, MA, USA); cyclin D1 and actin (clone ACTN05), from Neomarkers (Freemont, CA, USA); tubulin, from Sigma-Aldrich (St Louis, MO, USA); HRG (Ab-1), from Thermo Scientific; histone H3 and phospho-ErbB-3 (Tyr1289), from Abcam (Cambridge, MA, USA) and HRP-conjugated secondary antibody, from Vector Laboratories (Burlingame, CA, USA). The antibodies used for immunoprecipitation experiments, chromatin immunoprecipitation (ChIP) assays, and sequential ChIP assays were rabbit polyclonal anti-ErbB-2, anti-Stat3, and anti-ErbB-3 antibodies (C18, C-20, and C-17, respectively, from Santa Cruz Biotechnology); mouse monoclonal anti-HRG (Ab-1), from Thermo Scientific; CBP/KAT3A (ab2832) and acetyl histone H3 (06-599), from Millipore (Temecula, CA, USA). Rabbit IgG (Sigma-Aldrich) was used as negative control. Reagents Heregulin β1 (HRGβ1) was purchased from R&D Systems Inc (Minneapolis, MN, USA). Trastuzumab (Ttzm, HerceptinTM) was from Genentech Inc. (San Francisco, CA, USA). Tyrphostin AG825 and Jak inhibitor I were purchased from Calbiochem (San Diego, CA, USA). Western blots and immunoprecipitation assays The NE-PER Nuclear and Cytoplasmic Extraction Reagents technique (Pierce Biotechnology, Rockford, IL, USA) was performed according to manufacturer´s instructions. The use of this technique does not allow to obtain the cytoplasmic membrane fraction.1 Experiments in which phosphorylation levels of ErbB-2, ErbB-3, Stat3, and Akt were explored were repeated three to five times. Experiments assessing cyclin D1 and Stat3 protein levels were also repeated three to five times. Signal intensities of phospho-proteins were analyzed by densitometry and normalized to total protein bands. Similarly, signal intensities of cyclin D1 and Stat3 bands were normalized to actin bands. Data analysis in BT-474 and SK-BR-3 cells showed a significant decrease in pErbB-2 levels, and an increase in pErbB-3 and pAkt levels by HRGβ1 treatment in comparison with untreated cells, which remained unchanged when cells were transfected with hErbB-2ΔNLS (P<0.001). Ttzm treatment increased pErbB-2 levels, and decreased pErbB-3 and pAkt levels in both BT-474 and SK-BR-3 cells (P<0.001). Besides, HRGβ1 treatment increased pErbB-3 and pAkt levels in Ttzm-pretreated BT-474 and SK-BR-3 cells (P<0.001). HRGβ1 treatment induced a significant increase in pErbB-2, pErbB-3 and pAkt levels in JIMT-1 (P<0.001), also observed in cells transfected with hErbB-2∆NLS. Ttzm treatment increased pErbB-2 levels in JIMT-1 cells (P<0.001). HRGβ1 treatment also increased pErbB-3 and pAkt levels in JIMT-1 cells pretreated with Ttzm (P<0.001). Experiments in C4HD or T47D cells showed a significant increase in pErbB-2, pErbB-3, and pStat3 levels by HRGβ1 treatment in comparison with untreated cells, and a significant inhibition of HRGβ1induced protein phosphorylation when using the pharmacological inhibitor of ErbB-2 (AG825) or ErbB-2 siRNAs (P<0.001). Similar data analysis showed that compared with control cells, increase in cyclin D1 levels by HRGβ1 treatment from 12 to 72 h was significant, as was inhibition of HRGβ1 effects by hErbB-2∆NLS, ErbB-2 and Stat3 inhibitors and siRNAs (P<0.001). Differences between groups were analyzed by unpaired 2-tailed Student’s t test. Plasmids and transient transfections Human wild-type ErbB-2 expression vector (hErbB-2WT) as well as the empty pMe18SM vector were a gift from T. Yamamoto (University of Tokyo, Japan).2 The green fluorescent protein (GFP)-tagged human ErbB-2 mutant, which lacks the putative nuclear localization signal sequence (aa 676-KRRQQKIRKYTMRR-689), resulting in the sequence of KLM at the deletion junction (hErbB-2ΔNLS), was generously provided by M.C. Hung (The University of Texas M.D. Anderson Cancer Center, Houston, TX, USA).3 The empty pcDNA3.1 vector was a gift of J. Darnell. The empty pEGFP-N1 vector was obtained from BD Biosciences-Clontech (Palo Alto, CA, USA). Transfection efficiencies, evaluated using the pEGFP-N1 vector and determined by the percentage of cells that exhibited GFP 4 days after transfection, varied between 60–70%. siRNA sequences siRNAs targeting ErbB-2 and Stat3 were synthesized by Dharmacon, Inc. (Lafayatte, CO, USA). and their sequences were as follows: mouse ErbB-2 siRNA #1 5’GAUGGUGCUUACUCAUUGA-3’ and mouse ErbB-2 siRNA #2 5’- GAUGUCCUCCGUAAGAAUA-3’, both designed to specifically knock down mouse ErbB-2 but not human GGACGAAUUCUGCACAAUG-3’; GACGAAUUCUGCACAAUGG-3’; ErbB-2; human human Stat3 ErbB-2 ErbB-2 siRNA siRNA siRNA #1, #2, #1, 5’5’5’- GGUCAAAUUUCCUGAGUUGUU-3’; and Stat3 siRNA #2, 5’- GAGCAGAGAUGUGGGAAUGUU-3’. A nonsilencing siRNA oligonucleotide from Dharmacon that does not target any known mammalian gene was used as a negative control. In every case, experiments were performed with the two different siRNA sequences for each protein, but the results obtained with only one of them are presented here. Immunofluorescence and confocal microscopy in cell cultures ErbB-2 was localized using either a rabbit polyclonal (C-18) or a mouse monoclonal (F-11 or 9G6) ErbB-2 antibody (Santa Cruz Biotechnology). ErbB-2 F-11 was used in experiments evaluating the effect of hErbB-2∆NLS on endogenous ErbB-2 nuclear localization in murine C4HD cells, and ErbB-2 9G6 was used in experiments analyzing ErbB-2/ErbB-3 colocalization. Stat3 was detected using a mouse monoclonal antibody (124H6, Cell Signaling), ErbB-3 was localized using a rabbit polyclonal antibody (C-17, Santa Cruz Biotechnology), and HRG was detected using a rabbit polyclonal antibody (C-20, Santa Cruz Biotechnology). Secondary antibodies for ErbB-2 (C-18), ErbB-3 (C-17), and HRG (C-20) were goat anti-rabbit IgG-Alexa 488 (Molecular Probes, Eugene, OR, USA) or goat anti-rabbit IgG-rhodamine (Jackson ImmunoResearch Laboratories, West Grove, PA, USA); and for ErbB-2 (F-11 or 9G6) and Stat3, we used a rhodamine conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories). Negative controls were carried out using PBS instead of primary antibodies, or 5X competitive peptide (Santa Cruz Biotechnology) when ErbB-2 (C-18) was used. When cells were transfected with hErbB-2ΔNLS, green fluorescent protein (GFP) from this expression vector was visualized by direct fluorescence imaging. We performed quantitative analysis of confocal images with Image J (National Institutes of Health) to evaluate the percentages of ErbB-2 localized at the nucleus and the cytoplasmic membrane. Segmentation of the whole cell was performed using ErbB-2 images. The cytoplasmic membrane compartment was defined as the difference between the image of the cell and a binary erosion (iterations: 5-25), and the nuclear compartment was defined according to the DAPI images. We obtained an integrated fluorescence intensity value for total ErbB-2 (tErbB-2), for membrane ErbB-2 (mErbB-2), and for nuclear ErbB-2 (nErbB-2). Fluorescence background (median) was subtracted in all cases. To compute ErbB-2 cellular distribution, we calculated the ratio of integrated fluorescence intensities of mErbB-2/tErbB-2 (MErbB-2) and nErbB-2/tErbB-2 (NErbB-2) for 50 cells from each group and obtained an average value. Quantitative analysis of ErbB-2 subcellular localization is expressed as the percentage of MErbB-2 or NErbB-2, or as the ratio of MErbB-2/NErbB-2. Quantitative analysis of nuclear ErbB-3 (NErbB-3) levels in confocal images was performed as indicated for NErbB-2. In experiments where protein colocalization was evaluated, approximately 100-200 cells were analyzed for each treatment, out of which around 80% showed the same pattern of Stat3, ErbB-2, ErbB-3 and HRG cellular localization. Figures 2a, 2c, 3a, 4b, 4d, 4e, 4g-i, and 7a, and Supplementary Figures S1a, S1b, S2b, S2c, S2d, S3c, and S5 illustrate a few cells representative of the ones examined. DNA sequence of primers used for quantitative ChIP Mouse cyclin D1 amplicon containing two GAS sites (-971 and -874): 5’-TTCCGGTGGTCTGGTTCCT-3’ and 5’-GAGACACGATAGGCTCCTTCCTAA-3’. Human cyclin D1 amplicon containing one GAS site (-984): 5’-GGAACCTTCGGTGGTCTTGTC-3’ and 5’-GAATGGAAAGCTGAGAAACAGTGA3’. DNA sequence of primers used for quantitative RT-PCR Primers used for qPCR were designed with “Primer Express” software (Applied Biosystems, Foster City, CA, USA). Human cyclin D1 cDNA: 5'-TATTGCGCTGCTACCGTTGA-3' and 5'-CCAATAGCAGCAAACAATGTGAAA-3'. GAPDH cDNA: 5'-CCAGAACATCATCCCTGCAT-3' and 5'-GTTCAGCTCTGGGATGACCTT-3'. HRGβ1 pellet preparation and implantation HRGβ1 was encapsulated within Elvax pellets as described elsewere.4,5 A lyophilized mixture containing 100 μg of recombinant human HRG1 and 25 mg of bovine serum albumin (BSA) as a carrier was suspended in 125 μl ethylene acetate copolymer (Elvax 40W, generously donated by Simko, Bs As, Argentina) dissolved previously in dichloromethane (20% w/v). The mixture was snap-frozen in liquid nitrogen and dried under vacuum. The dried Elvax pellet was compressed between tweezers and cut to weight (1.0 mg = 5 μg HRGβ1). Mice were implanted subcutaneously with pellets using a trocar in the flank of cell inoculum. As control, Elvax pellet was prepared with BSA alone. Patients and Tissue Microarrays (TMAs) The Review Board on Human Research of Universidad de La Frontera (UF) reviewed and approved the collection of tumor specimens, our survey data, and all clinical and pathological information as well as the retrospective analyses on anonymized specimens from the Temuco Hospital archival cohort. We selected 42 membrane ErbB-2overexpressing paraffin-embedded tissue samples from a cohort of 291 archived invasive breast carcinomas from the files of the Histopathology Department of Temuco Hospital, Chile, from 1998 to 2006 (ref. 6). This cohort is composed by the 273 archived invasive breast carcinomas we previously published,6 plus 18 specimens from the original 346 consecutively archived invasive breast carcinomas from which we have recently obtained follow-up data. All retrospectively selected patients were treated with surgery. Follow-up data was available for up to 13 years with a median follow-up time of 53 months. Informed written consents were obtained from all patients before inclusion. Pre-treatment patient staging was classified according to the American Joint Committee on Cancer (AJCC) system7 through the Elston and Ellis histological grading system.8 TMAs were constructed at the UF TMA Core Facility as previously described.6 Immunofluorescence (IF) and immunohistochemistry (IHC) analysis of TMAs IF analysis of TMAs was performed as we described.6 Briefly, antigen retrieval was performed by immersing the sections in 10 mM sodium citrate buffer pH 6 and microwaving at high power for 4 min. Slides were blocked in Modified Hank’s Buffer (MHB) with 5% bovine serum albumin for 30 min and were incubated overnight at 4ºC with the following primary antibodies: polyclonal rabbit ErbB-2 antibody (C-18, Santa Cruz Biotechnology) and polyclonal rabbit Stat3 antibody (C-20, Santa Cruz Biotechnology). Slides were then incubated with anti-rabbit IgG-Alexa 488 antibody (Molecular Probes). Reduction of the autofluorescent background was performed by incubation with Sudan Black B 0.1% (Sigma-Aldrich). Nuclei were stained with DAPI (4’,6-diamidino-2-phenylindole). Slides were analyzed by a Nikon Eclipse E800 confocal laser microscopy system. Negative controls were carried out with MHB instead of primary antibodies. C4HD tumors from the model of mammary tumors induced by progestins were also used as controls.1 Slides were independently scored by two pathologists (PG and JCR). Score discrepancies were re-evaluated and reconciled on a two-headed microscope. Membrane ErbB-2 (MErbB-2) expression levels detected by IF were semiquantified using the same scores as those used in IHC staining (see below). Nuclear ErbB-2 and Stat3 (NErbB-2 and NStat3, respectively) levels detected by IF were scored considering both the percentage of positive cells and staining intensity. A score of 0 represents faint or no staining in less than 10% of cells, 1+ weak nuclear staining in 1025%, 2+ moderate staining in 26-50%, and 3+ strong staining in > 50% of cells. Scores of 2+ and 3+ were considered positive for NErbB-2 presence6 and scores of 1+, 2+ and 3+ were considered positive for NStat3 (ref. 9). NErbB-2 and NStat3 were evaluated in duplicate arrays. MErbB-2 was also evaluated by IHC with c-erb-B2 clone A0485 (Dako Carpinteria, CA, USA), as we already described.6 MErbB-2 was scored according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines.10 Scores 2+ in which FISH confirmed ErbB-2 amplification, and 3+ were considered positive for MErbB-2 overexpression.10 SUPPLEMENTARY REFERENCES 1. Beguelin W, Diaz Flaque MC, Proietti CJ, Cayrol F, Rivas MA, Tkach M et al. Progesterone receptor induces ErbB-2 nuclear translocation to promote breast cancer growth via a novel transcriptional effect: ErbB-2 function as a coactivator of Stat3. Mol Cell Biol 2010; 30: 5456-5472. 2. Akiyama T, Matsuda S, Namba Y, Saito T, Toyoshima K, Yamamoto T. The transforming potential of the c-erbB-2 protein is regulated by its autophosphorylation at the carboxyl-terminal domain. Mol Cell Biol 1991; 11: 833-842. 3. Giri DK, Ali-Seyed M, Li LY, Lee DF, Ling P, Bartholomeusz G et al. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol 2005; 25: 11005-11018. 4. Silberstein GB, Daniel CW. Reversible inhibition of mammary gland growth by transforming growth factor-beta. Science 1987; 237: 291-293. 5. Jones FE, Jerry DJ, Guarino BC, Andrews GC, Stern DF. Heregulin induces in vivo proliferation and differentiation of mammary epithelium into secretory lobuloalveoli. Cell Growth Differ 1996; 7: 1031-1038. 6. Schillaci R, Guzman P, Cayrol F, Beguelin W, Diaz Flaque MC, Proietti CJ et al. Clinical relevance of ErbB-2/HER2 nuclear expression in breast cancer. BMC Cancer 2012; 12: 74. 7. Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol 2002; 20: 3628-3636. 8. Page DL, Ellis IO, Elston CW. Histologic grading of breast cancer. Let's do it. Am J Clin Pathol 1995; 103: 123-124. 9. Dolled-Filhart M, Camp RL, Kowalski DP, Smith BL, Rimm DL. Tissue microarray analysis of signal transducers and activators of transcription 3 (Stat3) and phospho-Stat3 (Tyr705) in node-negative breast cancer shows nuclear localization is associated with a better prognosis. Clin Cancer Res 2003; 9: 594-600. 10. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013; 31: 39974013.