Example Cyclosporin Tacrolimus Consents
advertisement

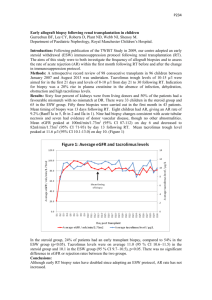
INFORMATION AND PATIENT CONSENT FOR TREATMENT OF CYCLOSPORIN General Information: Cyclosporin A has been used in the field of organ transplantation to prevent rejection of transplanted human organs. Numerous trials abroad and in the United States have shown its efficacy in the treatment of psoriasis, a common skin disease. The drug has not been approved for the therapy of chronic hives by the FDA, but there are numerous studies demonstrating effectiveness of cyclosporine in treating chronic hives. Cyclosporin A is only used in the treatment of chronic hives when other approved therapies have failed. WHAT WILL HAPPEN TO YOU DURING TREATMENT: Baseline laboratory evaluations will be obtained which may include blood cell counts, blood chemistry, liver function, kidney function, pregnancy test if appropriate, and further tests or medical evaluations deemed necessary by our physicians. A specific test of kidney function (creatinine) will be done at regular intervals by checking the blood chemistry. FOR FEMALES: CYCLOSPORIN HAS NOT BEEN DEEMED SAFE IN PREGNANCY AND IS NOT SAFE FOR BREAST-FEEDING WOMEN: Cyclosporin has consistently been associated with premature birth and low birth-weight babies. If you are female and you are physically capable of becoming pregnant, you must agree to use a reliable method of birth control. All females who are pre-menopausal and not surgically sterilized (by either hysterectomy or tubal ligation) are considered to be physically capable of becoming pregnant. We must have your agreement to use a reliable method of birth control, and your signing of this form means that you agree to use a reliable method of birth control. We define the term “reliable method” of birth control as: A) a “systemic” form of birth control that is followed as exactly outlined the specific birth control instructions. These birth control methods include either the oral contraceptive pill, contraceptive patch, Intra-Uterine Device (aka “IUD”), depotprogesterone (aka “Depot”), or intravaginal hormonal contraceptive (aka “NuvaRing”) or B) “Double-barrier method”—which means condom use for the man AND diaphragm or female condom for the woman. Both devices must be used exactly as outlined in the instructions. By signing this form, you are stating that you understand what our definition “reliable birth control” is and that you agree to using a reliable form of birth control, and that you understand that cyclosporine has been shown to be dangerous in pregnancy by leading to premature births and low-birth-weight babies. The physician who prescribes cyclosporin is in no way ethically or legally responsible for any adverse effects to the fetus that may occur if you get pregnant while on cyclosporine; and, by signing this document, you are stating that you have read and understand this statement that the prescribing physician is not responsible. You cannot use cyclosporin when breast-feeding. Cyclosporin in breast milk may harm the baby, so you cannot use cyclosporine when breast-feeding. DIETERY RESTRICTION: You cannot drink grapefruit juice. Grapefruit juice can increase blood levels of cyclosporine, therefore increasing the risk of cyclosporin toxicity. Avoid taking any over-the-counter herbal medications. Any herbal medicine may interact with the metabolism of cyclosporin. Specifically, do not take St. John’s Wort. St. John’s Wort decreases blood levels of cyclosporin, thereby decreasing its therapeutic effectiveness. FACTS YOU SHOULD KNOW: Cyclosporin causes many side effects, and the kidney is one of the more common organs that are affected. For this reason, we will closely monitor blood pressure, blood tests, kidney function tests, and medical findings that related to kidney function. The dose of cyclosporin may be periodically adjusted depending upon your physical exam and lab tests to minimize the potential for adverse effects. There is a BOX-WARNING from the FDA regarding the increased susceptibility to both infection and malignancy, mainly lymphoma, in patients taking tacrolimus. Of note, the studies that showed this only included organ-transplant patients who were also taking other immunosuppressive drugs in-addition to tacrolimus, and it has been a well-known fact for many years that post-organ transplant patients on multiple immunosuppressents are at increased risk for both infection and malignancy, mainly lymphoma. This is the FDA Box-Warning: WARNING : Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe Cyclosporine Capsules USP NONMODIFIED. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient. Cyclosporine Capsules USP NON-MODIFIED should be administered with adrenal corticosteroids but not with other immunosuppressive agents. Increased susceptibility to infection and the possible development of lymphoma may result from immunosuppression. Cyclosporine Soft Gelatin Capsules USP (NON-MODIFIED) have decreased bioavailability in comparison to Neoral®* (cyclosporine capsules, USP) MODIFIED. Cyclosporine Capsules USP (NON-MODIFIED) and Neoral® (cyclosporine capsules, USP) MODIFIED are not bioequivalent and cannot be used interchangeably without physician supervision. The absorption of cyclosporine during chronic administration Cyclosporine Capsules USP NON-MODIFIED was found to be erratic. It is recommended that patients taking Cyclosporine Capsules USP NON-MODIFIED over a period of time be monitored at repeated intervals for cyclosporine blood levels and subsequent dose adjustments be made in order to avoid toxity due to high levels and possible organ rejection due to low absorption of cyclosporine. This is of special importance in liver transplants. Numerous assays are being developed to measure blood levels of cyclosporine. Comparison of levels in published literature to patient levels using current assays must be done with detailed knowledge of the assay methods employed. Cyclosporin rarely causes increased blood levels of the liver enzymes, which reflects liver inflammation. This inflammatory effect on the liver is an idiosyncratic reaction and not dependent on the dose of cyclosporin. Caution with consumption of alcohol is required, so you should not drink excessive amounts of alcohol. Levels of uric acid may increase and provoke flares of gout if you have a predisposition for having gout. If you have a history of gout, you must inform us of this history. In addition to potential kidney and liver toxicity, adverse reactions such as tremor, increased hair growth, and excessive growth of the gums occurred in 3% or greater of the patients involved in clinical trials of kidney, heart, and liver transplants. There is also an increased risk of skin tumors and, less frequently, other tumors. However, these adverse effects are less likely at the low doses of cyclosporin A used for chronic hives and swelling (aka urticaria and angioedema), and these side effects of often reversible or treatable. Other reactions that occur in 2% or less of patients include allergic reactions, anemia, decreased appetite, confusion, eye inflammation, swelling, fever, brittle fingernails, stomach irritation, hearing loss, hiccups, elevated blood sugars, muscle pain, peptic ulcers, decreased platelet counts, and ringing of the ears. Other reactions that occur rarely include: anxiety, chest pain, constipation, depression, hair breakage, blood in the urine, joint pain, lethargy, mouth sores, heart attack, night sweats, pancreatitis, itching, swallowing difficulties, tingling, stomach bleeding, visual disturbances, weakness, and weight loss. You will be very carefully followed by our physicians to monitor any potential side effects, both by examination as well as evaluation of laboratory test results. PARTICIPATION IN THIS THERAPY: To participate in this treatment, we will need your complete cooperation. Do not take any prescription or over-the-counter drugs or medications without notifying our staff because there are many drugs that interact with cyclosporin. Please inform all physicians that you are being treated with cyclosporine at each visit. You should avoid excessive sun exposure. If you are female, you should have a PAP smear every year while on this medication. Bring any new or changing skin lesions to our attention. Please notify us of any swellings or enlarged glands anywhere underneath your skin when you are seen for your regular exams. We will absolutely discontinue your treatment with this drug if you fail to follow instructions, fail to return for your appointments, or develop significant side effects. In this regard, it is essential that you ensure that all laboratory tests are done in a timely fashion. If we do not receive your results, we will be unable to continue your treatment. If you have any questions or problems concerning your treatment with this drug, or if you feel you are experiencing side effects, or if you begin taking another drug, please contact our staff immediately at _________. SUMMARY: 1) Follow the instructions given to you by the physicians at our clinic. If you have any questions, please do not hesitate to call our office. 2) Please monitor your blood pressure as instructed. 3) Do not fail to get blood tests as instructed. You should have the results sent to us promptly. Our fax number is _________. 4) Do not miss appointments. 5) Inform us of any new medications that have been prescribed for you. 6) Please contact us immediately if you feel you are experiencing any side effects. I HAVE READ AND UNDERSTAND ALL OF THE ABOVE INFORMATION AND INSTRUCTIONS, AND I GIVE MY VOLUNTARY CONSENT TO PARTICIPATE. Patient’s Signature:_______________________ Date_____________ Witness’ Signature:_______________________ Date_____________ INFORMATION AND PATIENT CONSENT FOR TREATMENT OF TACROLIMUS General Information: Tacrolimus has been used in the field of organ transplantation to prevent rejection of transplanted human organs. The drug has not been approved for the therapy of chronic hives by the FDA, but there is a study demonstrating effectiveness of tacrolimus in treating chronic hives/angioedema. Tacrolimus is only used in the treatment of chronic hives when other approved therapies have failed. WHAT WILL HAPPEN TO YOU DURING TREATMENT: Baseline laboratory evaluations will be obtained which may include blood cell counts, blood chemistry, liver function, kidney function, pregnancy test if appropriate, and further tests or medical evaluations deemed necessary by our physicians. A specific test of kidney function (creatinine) will be done at regular intervals by checking the blood chemistry. FOR FEMALES: TACROLIMUS HAS NOT BEEN DEEMED SAFE IN PREGNANCY AND IS NOT SAFE FOR BREAST-FEEDING WOMEN: Tacrolimus has consistently been associated with premature birth and low birth-weight babies. If you are female and you are physically capable of becoming pregnant, you must agree to use a reliable method of birth control. All females who are pre-menopausal and not surgically sterilized (by either hysterectomy or tubal ligation) are considered to be physically capable of becoming pregnant. We must have your agreement to use a reliable method of birth control, and your signing of this form means that you agree to use a reliable method of birth control. We define the term “reliable method” of birth control as: A) sexual abstinence, the 100%-reliable form of birth control OR B) a “systemic” form of birth control that is followed as exactly outlined the specific birth control instructions. These birth control methods include either the oral contraceptive pill, contraceptive patch, Intra-Uterine Device (aka “IUD”), depotprogesterone (aka “Depot”), or intravaginal hormonal contraceptive (aka “NuvaRing”) OR C) “Double-barrier method”—which means condom use for the man AND diaphragm or female condom for the woman. Both devices must be used exactly as outlined in the instructions. By signing this form, you are stating that you understand what our definition “reliable birth control” is and that you agree to using a reliable form of birth control, and that you understand that tacrolimus has been shown to be dangerous in pregnancy by leading to premature births and low-birth-weight babies. The physician who prescribes tacrolimus is in no way ethically or legally responsible for any adverse effects to the fetus that may occur if you get pregnant while on tacrolimus because you have been fully warned by this document that tacrolimus may harm the fetus and newborn baby. By signing this document, you are stating that you have read and understand this statement that the prescribing physician at Redding Allergy and Center is not in any way responsible for any harm that may occur to the fetus/baby. You cannot take tacrolimus if you are breast-feeding., because tacrolimus excreted in breast milk may harm the baby. DIETERY RESTRICTION: You cannot drink grapefruit juice. Grapefruit juice can increase blood levels of tacrolimus, therefore increasing the risk of tacrolimus toxicity. Avoid taking any over-the-counter herbal medications. Any herbal medicine may interact with the metabolism of tacrolimus. Specifically, do not take St. John’s Wort. St. John’s Wort decreases blood levels of tacrolimus, thereby decreasing its therapeutic effectiveness. FACTS YOU SHOULD KNOW: Tacrolimus can cause many side effects, and the kidney is one of the more common organs that are affected. For this reason, we will closely monitor blood pressure, blood tests, kidney function tests, and medical findings that related to kidney function. The dose of tacrolimus may be periodically adjusted depending upon your physical exam and lab tests to minimize the potential for adverse effects. Tacrolimus increases the chance of developing new-onset diabetes mellitus as well as worsening sugar (aka glucose) control in patients with existing diabetes mellitus. Therefore, you must notify our office as well as your primary care doctor if you have ANY increase in thirst, urinary frequency or amount, hunger, or fatigue, which are clues that you have diabetes or worsening glucose control. There is a BOX-WARNING from the FDA regarding the increased susceptibility to both infection and malignancy, mainly lymphoma, in patients taking tacrolimus. Of note, the studies that showed this only included organ-transplant patients who were also taking other immunosuppressive drugs in-addition to tacrolimus, and it has been a well-known fact for many years that post-organ transplant patients on multiple immunosuppressents are at increased risk for both infection and malignancy, mainly lymphoma. This is the FDA Box-Warning: “Warning—Increased susceptibility to infection and the possible development of lymphoma may result from immunosuppression. Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe tacrolimus. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient” Tacrolimus rarely causes increased blood levels of the liver enzymes, which reflects liver inflammation. This inflammatory effect on the liver is an idiosyncratic reaction and not dependent on the dose of tacrolimus. Caution with consumption of alcohol is required, so you should not drink excessive amounts of alcohol. Levels of uric acid may increase and provoke flares of gout if you have a predisposition for having gout. If you have a history of gout, you must inform us of this history. In addition to potential kidney and liver toxicity, adverse reactions such as tremor, increased hair growth, and excessive growth of the gums occurred in 3% or greater of the patients involved in clinical trials of kidney, heart, and liver transplants. There is also an increased risk of skin tumors and, less frequently, other tumors. However, these adverse effects are less likely at the low doses of tacrolimus used for chronic hives and swelling (aka urticaria and angioedema), and these side effects of often reversible or treatable. Other reactions that occur in 2% or less of patients include allergic reactions, anemia, decreased appetite, confusion, eye inflammation, swelling, fever, brittle fingernails, stomach irritation, hearing loss, hiccups, elevated blood sugars, muscle pain, peptic ulcers, decreased platelet counts, and ringing of the ears. Other reactions that occur rarely include: anxiety, chest pain, constipation, depression, hair breakage, blood in the urine, joint pain, lethargy, mouth sores, heart attack, night sweats, pancreatitis, itching, swallowing difficulties, tingling, stomach bleeding, visual disturbances, weakness, and weight loss. You will be very carefully followed by our physicians to monitor any potential side effects, both by examination as well as evaluation of laboratory test results. PARTICIPATION IN THIS THERAPY: To participate in this treatment, we will need your complete cooperation. Do not take any prescription or over-the-counter drugs or medications without notifying our staff because there are many drugs that interact with tacrolimus. Please inform all physicians that you may see that you are being treated with tacrolimus at each visit. You should avoid excessive sun exposure. If you are female, you should have a PAP smear every year while on this medication, because if you there are any cervical changes concerning for cancer or pre-cancer, cyclosporine may need to be stopped. Also, bring any new or changing skin lesions to our attention. Please notify us of any swellings or enlarged glands anywhere underneath your skin when you are seen for your regular exams. We will absolutely discontinue your treatment with this drug if you fail to follow instructions, fail to return for your appointments, or develop significant side effects. In this regard, it is essential that you ensure that all laboratory tests are done in a timely fashion. If we do not receive your results, we will be unable to continue your treatment. If you have any questions or problems concerning your treatment with this drug, or if you feel you are experiencing side effects, or if you begin taking another drug, please contact our staff immediately at 404-355-0078. SUMMARY: 1) Follow the instructions given to you by the physicians at our clinic. If you have any questions, please do not hesitate to call our office. 2) Please monitor your blood pressure as instructed. 3) Do not fail to get blood tests as instructed. You should have the results sent to us promptly. Our fax number is _________. 4) Do not miss appointments. 5) Inform us of any new medications that have been prescribed for you. 6) Please contact us immediately if you feel you are experiencing any side effects. I HAVE READ AND UNDERSTAND ALL OF THE ABOVE INFORMATION AND INSTRUCTIONS, AND I GIVE MY VOLUNTARY CONSENT TO PARTICIPATE. Patient’s Signature:_______________________ Date_____________ Witness’ Signature:_______________________ Date_____________