BIO 220 Laboratory Exercises
advertisement

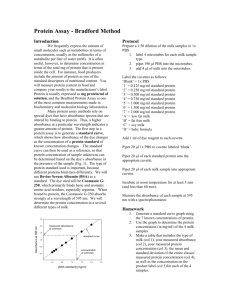
BIO 220 Laboratory Exercises Introduction to Laboratory Techniques This exercise consists of three parts: 1) Use of a pH meter and preparation of homogenization media 2) Use of the UV-VIS spectrophotometer 3) Preparation of a standard curve for protein quantitation These three tasks are commonly performed in cell and molecular biology research laboratories. Part 1. Use of a pH meter and preparation of homogenization media Upon completion of this exercise, students will be able to: - Standardize and use a pH meter - Prepare a biological buffer and adjust the pH In the next lab exercise, mitochondria will be isolated from rat liver using homogenization media during disruption of the cells. A goal of today's exercise is to prepare this solution. The media contains 0.25 M sucrose, 1 mM ethylenediaminetetraacetic acid (EDTA), and 50 mM Tris-HCl, pH 7.4. Each group of 3 students must prepare 200 mL of homogenization media for use at the next lab meeting. The pH meter is essentially a voltmeter designed to measure the potential difference between a reference electrode and a sensing electrode. The reference electrode usually contains a solution of AgCl2, and the solution to be tested contains varying amounts of protons (H+). The instrument measures the difference in electrical potential (mV) between these solutions and translates this into a pH reading. Obtaining an accurate reading with a pH meter depends on effective standardization, static charge, solution temperature, and other factors. Generally, it is desirable to calibrate the meter with a standard buffer of a pH as close as possible to the pH of the solution to be measured. The problem of static charge can be minimized by blotting, not wiping, the electrode gently with a Kimwipe before transferring to a new solution. The temperature of the calibration solution should be as close as possible to the desired working temperature of the solution to be tested. Safety Notice Concentrated solutions of acids and bases will irritate eyes and skin. Be careful not to get these solutions in your eyes or on your skin. Procedure 1) Standardize the pH meter with pH 7 buffer. 2) Weigh the correct amount of Tris-base or Tris-HCl (M.W. = 121.1 g/mol) to make 200 mL of 50 mM buffer. Weigh proper amounts of sucrose (M.W. = 342.2 g/mol) and EDTA (M.W. = 372.2 g/mol) to yield final concentrations of 0.25 M and 1 mM, respectively, in 200 mL. 3) Dissolve the solids in approximately 150 mL of distilled water. This allows room for addition of acid or base to adjust the pH. Record the initial pH of the solution and titrate to pH 7.4 with either 1 M HCl or 1 M NaOH, as needed. 4) Transfer the solution to a graduated cylinder. Add distilled water for a final volume of 200 mL. You may wish to recheck the pH of the solution. Part 2. Use of the UV-VIS spectrophotometer Upon completion of this exercise, students will be able to: - Properly use variable-volume micropipettors - Prepare simple dilutions of a stock solution (i.e., 1/5, 1/10, etc.) - Use the UV-VIS spectrophotometer to record absorbance values - Generate a graph for determination of an extinction coefficient () The colorimeter (spectrophotometer) is an instrument that measures the amount of light absorbed by molecules in solution. The specimen is placed in a special glass or plastic cuvette. Light of a given wavelength is passed through the sample, and exiting light is measured by a phototube (detector). These machines automatically display the amount of light absorbed as absorbance (optical density) values. Absorbance is a unitless quantity, and is calculated by the following relationship: Where A= absorbance, Io = intensity of light entering the cuvette, and IT = intensity of light exiting the cuvette and reaching the detector. A number of factors will affect the light absorbed by a given substance including: concentration, the solvent used, the path length, and the characteristics of the cuvette. It is possible to determine the absorbance of a substance and calculate the absorptivity coefficient (). These coefficients are useful because they can be used to determine the concentration of a substance under defined conditions. The equation used to determine concentration is call the Beer-Lambert equation: Where l = path length, and c= concentration. In this lab, you will lean how to use the spectrophotometer, test your ability to accurately dilute a dye, and determine the absorptivity coefficient (, also called the extinction coefficient) of dichlorophenolindophenol (DCIP), an electron acceptor that we will use in future laboratory periods. Procedure 1) Turn on the spectrophotometer using the switch on the back right hand side. Make sure the instrument has been on at least 10 min before use. 2) Set the wavelength to 600 nm by using the keypad and "Go to ". 3) Prepare 4 dilutions of 0.5 mM dichlorophenolindophenol (DCIP) dye: 1/5, 1/10, 1/50, and 1/100. The dilutions may be prepared directly in plastic cuvettes (3 mL total volume). Use distilled water as the diluent. Prepare an additional "blank" cuvette that contains only water. 4) Zero the spectrophotometer. There are different methods available for "zeroing", "blanking", or "background-correcting" a spectrophotometer. In this course, an air-air background correction will always be used. Make sure there are NO CUVETTES in the instrument. Close the cover. Check that the instrument is set at the desired wavelength. Press the "Auto Zero" key. Once the display reads "0.000", the instrument is ready to use. 5) Place the blank cuvette containing water in the sample holder towards the back of the instrument, and one diluted dye sample in the sample holder in the front. Close the lid and record the absorbance at 600 nm. 6) Measure the absorbance (600 nm) for every diluted sample. Construct a graph of concentration versus absorbance. If your dilutions are perfect, a linear relationship will result. Determine "", the slope, from the graph. Part 3. Preparation of a standard curve for protein quantitation Upon completion of this exercise, students will be able to: - Dilute bovine serum albumin (BSA) for use as a standard in protein quantitation - Prepare a standard curve for the Bio-Rad Protein Assay, using BSA as the standard - Graph data for the standard curve, and observe the linear relationship between amount of protein and A595 nm. The Bio-Rad Protein Assay is a dye-binding assay based on the differential color change of the dye in response to different protein concentrations. This is a colorimetric assay, and the amount of protein in the unknown sample is determined by comparing the absorbance of the unknown sample to the concentration-versus-absorbance relationship defined by the standard curve. The assay uses Coomassie Brilliant Blue G-250 dye, and is based on a method developed by Dr. Marion Bradford at the University of Georgia1. The Bio-Rad assay is linear between 20 and 140 µg protein in a 0.1 mL sample. A sample standard curve, as reproduced from the technical literature for the Bio-Rad Protein Standard Assay2, is shown in Figure 1. The standard curve and sample points should be prepared at the same time. Absorbance readings (A595 nm) must be recorded 5 min to 1 hr after addition of the dye reagent. A new sample curve must be prepared each time the assay is run. Figure 1. This is an example of a Bio-Rad Protein Assay standard curve, reproduced from the technical literature2. Safety Notice Bio-Rad Dye contains phosphoric acid, and will stain skin and clothing. Avoid getting the dye in your eyes, on your skin, or on your clothes. Procedure 1) Each group will be provided with 200 µL of a 1.4 mg/mL BSA standard. This is enough material to complete the exercise, provided that good pipetting technique is used. 2) Prepare the standard curve using clean, dry, glass test tubes. 3) Make a table showing how much BSA standard and water to combine to make 0.1 mL of the following concentrations of BSA: 140, 70, 35, 17.5, and 0 µg/µL. 4) Following the table prepared in step 3, add the proper amounts of water and BSA standard to each glass tube. Important: Use a fresh tip for each transfer of BSA. 5) Use the bottle-top dispenser to add 5 mL of Bio-Rad Dye to each tube. Place a small piece of Parafilm over the tube, and vortex to mix. 6) Transfer (by pouring) solutions into plastic disposable cuvettes. Make sure cuvettes are filled to the mark. 7) Use the spectrophotometer to record the absorbance at 595 nm. The cuvette containing 0 µg/µL BSA should be used as the reference. References 1) Bradford, M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal. Biochem. 72, 248-254. 2) Bio-Rad Laboratories, Hercules, CA. (1999) Instruction Manual, Bio-Rad Protein Assay, Rev C., Bulletin Number LIT33, p. 5.