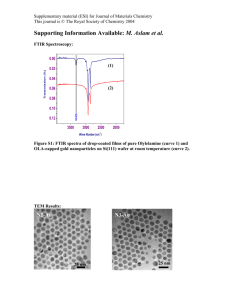
Further experimental and structural details
advertisement

Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 “On-off-on” fluorescent indicators of pH windows based on three separated components P. Pallavicini, V. Amendola, C. Massera, E. C. Mundum, A. Taglietti Supporting Information (Pages 16 including top sheet) Table SI Crystal data of [Cu(tcq)Cl]CF3SO3·1.5H2O pp 2 Table SII Table SIII Table SIV Table SV Figure SI Figure SII Figure SIII Figure SIV Figure SV Figure SVI Atomic coordinates of non-H atoms of [Cu(tcq)Cl]CF3SO3·1.5H2O pp 4 Anisotropic coefficients of non-H atoms of [Cu(tcq)Cl]CF3SO3·1.5H2O pp 5 Atomic coordinates of H atoms of [Cu(tcq)Cl]CF3SO3·1.5H2O pp 6 Listing of bond lengths (Å) and angles (deg) of [Cu(tcq)Cl]CF3SO3·1.5H2O pp 7 Molecular structure of [Cu(tcq)Cl]CF3SO3·1.5H2O pp 9 distribution diagram (% of species vs pH) for 1:1 tcq/Cu2+ pp 10 2+ distribution diagram (% of species vs pH) for 1:1 ccq/Cu pp 11 titration profile (emf vs ml of added base) for tcq plus excess acid pp 12 titration profile (emf vs ml of added base) for ccq plus excess acid pp 13 titration profile (emf vs ml of added base) for tcq plus 1:1 Cu2+ plus excess acid pp 14 Figure SVII titration profile (emf vs ml of added base) for ccq plus 1:1 Cu2+ plus excess acid pp 15 Figure SVIII emission spectra recorded by pH variation for Coumarine 343 pp 16 In the potentiometric and spectrophotometric experiments, samples of pure ligands were used, prepared by redissolution of the ammonium salts in 0.1 M NaOH, followed by extraction with CH2Cl2, drying over MgSO4, removal of dessicant and solvent, and highvacuum treatment (<0.1 Torr). Titrations were carried out on solutions of dioxane-water (4 : 1 v/v) with 0.1 M KNO3 as the background electrolyte. Cu(CF3SO3)2 was used as the copper salt. EMF vs. volume of added base profiles are provided. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Table SI. Crystal data of [Cu(tcq)Cl]CF3SO3·1.5H2O Formula fw Crystal system Space group a, Å b, Å c, Å , deg V, Å3 Z calcd, g cm-3 , mm-1 F(000) Crystal size, mm3 range, deg Limiting indices Reflns collected Independent reflns C27H28ClCuF3N4O3S·1.5H2O 671.61 Monoclinic C2 29.723(5) 8.506(5) 11.882(5) 94.33(1) 2995(2) 4 1.489 0.948 1384 0.22 x 0.15 x 0.28 1.37 to 27.03 -35h37, -10k10, -15l10 10133 6368 (Rint = 0.0494) Obs. reflns [I>2(I)] Data / restr. / param. 2777 6368 / 1 / 379 Goodness-of-fit on F2 0.610 Final R indices [I>2(I)] R indices (all data) Largest diff. peak and hole, eÅ-3 R1 = 0.0492, wR2 = 0.1038 R1 = 0.1355, wR2 = 0.1316 0.329 and -0.193 ^-3^M R1 = ||Fo|-|Fc||/| |Fo| wR2 = [[w(Fo2-Fc2)2/w(Fo2)2]1/2 Intensity data and cell parameters were recorded at room temperature (25°C) on a Bruker AXS Smart 1000 single-crystal diffractometer (MoK radiation) equipped with a CCD area detector, and automatically corrected for absorption using “SMART” software. The structure was solved by Patterson using the SHELXS-97 program [A] and refined on F2 by full-matrix least-squares using the SHELXL-97 program [A]. All non-hydrogen atoms were refined with anisotropic atomic displacements, while the hydrogen atoms were introduced into the geometrically calculated positions and refined “riding” on the corresponding parent atoms. The weighting scheme used in Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 the last cycle of refinement was w = 1/ [2Fo2 + (0.0837P)2] where P = (Fo2 + 2Fc2)/3. Molecular geometry calculations were carried out using the PARST97 program [B]. Drawings were obtained by ORTEP3 in the WinGX suite [C]. All calculations were carried out on a DIGITAL Alpha Station 255 computer. [A] G. M. Sheldrick, SHELX-97, Program for Crystal Structure Refinement, University of Göttingen, 1997; http://shelx.uni-ac.gwdg.de/shelx/index.html. [B] M. Nardelli, PARST97, updated version of PARST95, J. Appl. Crystallogr., 1995, 28, 659. [C] Ortep3 in the WinGX suite, L.J. Farrugia, J. Appl. Crystallogr., 1997, 30, 565. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Table SII. Fractional Atomic Coordinates (x104) and Equivalent Isotropic Thermal Parameters (Å2x104) with E.s.d.'s in Parentheses for [Cu(tcq)Cl]CF3SO3·1.5H2O. Atom Cu S Cl N1 N2 N3 N4 F1 F2 F3 O1 O2 O3 C1 C2 C3 C4 C5 C6 C7 C8 C9 C10 C11 C12 C13 C14 C15 C16 C17 C18 C19 C20 C21 C22 C23 C24 C25 C26 C27 O1S O2S X/a 6087.5(2) 6069.5(6) 6154.9(6) 6115(2) 5716(1) 5909(2) 6743(2) 6935(2) 6626(2) 6632(2) 6095(2) 6114(2) 5727(2) 5442(2) 5237(2) 4992(2) 5294(2) 5489(2) 5740(2) 5466(2) 5776(2) 5721(2) 6006(2) 6374(2) 6421(2) 6786(2) 7091(2) 7056(2) 6706(2) 6284(2) 6732(2) 7120(3) 7522(3) 7552(2) 7145(2) 7158(2) 7555(2) 7954(2) 7953(2) 6578(3) 5441(2) 5000(0) Y/b 5173.4(8) 6884.2(24) 3482.0(21) 3528(6) 6300(6) 6983(6) 6528(5) 6714(8) 4589(7) 5454(9) 7152(8) 8253(7) 5829(8) 7517(7) 8719(8) 9996(10) 10760(8) 9579(8) 8263(7) 5082(9) 3712(7) 2714(8) 1502(8) 1281(7) 2335(6) 2154(7) 983(8) -35(9) 91(9) 7443(9) 7331(7) 8046(8) 7881(9) 7054(8) 6416(7) 5660(7) 5487(9) 6058(10) 6854(9) 5838(12) 4031(12) 5799(10) Z/c 3260.6(5) -1215.2(19) 4747.5(15) 2007(4) 2025(4) 4232(4) 3563(4) -1091(6) -794(6) -2440(4) -49(5) -1858(6) -1580(6) 2528(5) 1696(6) 2267(7) 3205(7) 4023(6) 3460(5) 1371(5) 1204(5) 296(6) 181(5) 992(5) 1904(5) 2718(5) 2595(6) 1694(6) 911(6) 5021(6) 4511(5) 5038(5) 4586(6) 3587(6) 3071(5) 2034(5) 1548(6) 2076(7) 3050(7) -1408(8) 6626(7) 5000(0) Ueq 527(2) 808(8) 732(7) 479(16) 489(17) 585(18) 510(17) 1484(30) 1468(30) 1534(29) 1238(28) 1281(30) 1311(31) 538(21) 708(26) 877(29) 838(32) 773(29) 558(21) 618(20) 495(20) 640(25) 645(25) 550(22) 496(21) 594(23) 687(26) 700(26) 686(23) 772(30) 549(22) 706(27) 725(28) 637(25) 531(21) 603(24) 818(30) 874(32) 790(30) 909(36) 1827(42) 1539(49) Equivalent Isotropic U defined as one-third of the race of the orthogonalized Uij tensor. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Table SIII. Anisotropic Thermal Parameters(x104) with E.s.d.'s in parentheses for the non-Hydrogen Atoms of [Cu(tcq)Cl]CF3SO3·1.5H2O.They are in the form: xp(-22(U11h2a*2+...+2U12hka*b*+...)) Atom Cu S N1 N2 N3 N4 F1 F2 F3 O1 Cl O2 O3 C1 C2 C3 C4 C5 C6 C7 C8 C9 C10 C11 C12 C13 C14 C15 C16 C17 C18 C19 C20 C21 C22 C23 C24 C25 C26 C27 O1S O2S U11 542(4) 792(13) 474(26) 419(26) 606(31) 502(28) 752(31) 1486(49) 1705(46) 1522(51) 868(12) 1255(47) 839(34) 411(31) 544(37) 683(39) 749(46) 729(46) 439(31) 453(28) 466(32) 565(39) 686(41) 605(37) 557(34) 622(38) 735(44) 726(41) 735(38) 891(55) 675(40) 1048(58) 699(45) 613(42) 510(35) 502(34) 769(45) 592(45) 487(40) 900(60) 1188(48) 1704(81) U22 549(4) 761(14) 475(29) 561(30) 636(33) 543(32) 1559(52) 1102(47) 2038(68) 1301(51) 752(12) 1121(49) 1558(68) 572(38) 702(46) 650(45) 526(42) 696(49) 581(38) 713(38) 546(37) 735(46) 690(47) 534(38) 437(36) 548(40) 505(37) 573(47) 476(34) 873(57) 465(36) 561(41) 809(51) 564(40) 513(36) 659(49) 781(60) 1126(61) 947(57) 950(61) 2422(91) 1106(72) U33 U23 485(4) -34(5) 889(15) -107(12) 486(28) -4(24) 492(29) 0(25) 522(32) -65(28) 479(30) -97(25) 2156(67) -36(50) 1822(57) 437(42) 895(33) -183(42) 982(43) -435(38) 574(11) 110(9) 1516(57) 377(45) 1500(55) -561(46) 645(40) 54(33) 858(49) 128(40) 1305(62) 83(55) 1240(70) -130(44) 928(54) -43(41) 671(41) 33(34) 673(37) -113(45) 467(35) -6(31) 603(43) -114(37) 557(42) -84(35) 515(37) 0(32) 503(37) 44(29) 591(40) -39(32) 809(51) 85(37) 807(46) 39(43) 873(46) -155(49) 537(43) -153(39) 487(37) -21(30) 466(38) -52(31) 627(47) 0(42) 710(47) 146(37) 563(38) 13(30) 651(40) -55(32) 924(50) -88(42) 918(59) 50(52) 911(57) 115(49) 908(64) 104(55) 1900(73) -1016(71) 1971(99) 0(0) U13 U12 -6(3) 184(12) 20(24) 60(23) 109(27) -14(24) 208(37) 156(45) 329(33) 686(39) 42(10) 423(43) -155(34) 129(30) -72(35) 107(42) 85(48) 288(42) 160(31) -51(27) -4(30) -67(33) 40(36) 69(32) 95(30) -92(34) -25(39) 102(37) 222(36) -36(41) -90(32) -228(41) -231(43) -97(37) -11(31) 51(31) 197(41) 149(44) -116(41) 269(53) 296(51) 1215(78) 26(5) 61(11) -62(25) -29(24) 11(28) -11(24) 71(35) 382(35) 837(50) -355(43) 79(11) 474(41) -30(37) -40(29) 27(36) 160(47) 62(35) 58(36) 35(30) 61(42) -84(30) -101(36) -94(37) -140(33) -47(31) -5(33) 44(37) 17(42) -38(45) 88(43) -27(31) -38(40) -106(38) -57(33) -49(28) -97(29) -149(42) -95(43) -166(38) 45(50) -350(54) 0(0) Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Table SIV. Fractional Atomic Coordinates (x104) and Isotropic Thermal Parameters (x104) with E.s.d.'s in parentheses for the Hydrogen Atoms in [Cu(tcq)Cl]CF3SO3·1.5H2O. Atom H1 H2 H3 H2A H2B H3A H3B H4A H4B H5A H5B H6 H7A H7B H9 H10 H13 H14 H15 H16 H17A H17B H19 H20 H23 H24 H25 H26 X/a 5194(0) 5906(0) 5678(0) 5474(0) 5028(0) 4727(0) 4892(0) 5536(0) 5119(0) 5248(0) 5696(0) 5999(0) 5353(0) 5210(0) 5483(0) 5962(0) 6819(0) 7330(0) 7274(0) 6681(0) 6290(0) 6239(0) 7101(0) 7780(0) 6893(0) 7559(0) 8225(0) 8221(0) Y/b 6988(0) 6780(0) 6660(0) 9183(0) 8193(0) 9559(0) 10787(0) 11321(0) 11518(0) 9125(0) 10102(0) 8729(0) 5500(0) 4737(0) 2875(0) 817(0) 2828(0) 868(0) -808(0) -611(0) 6772(0) 8516(0) 8628(0) 8325(0) 5270(0) 4982(0) 5886(0) 7278(0) Z/c 2874(0) 1569(0) 4637(0) 1288(0) 1153(0) 2582(0) 1716(0) 2877(0) 3599(0) 4425(0) 4571(0) 3122(0) 644(0) 1769(0) -247(0) -428(0) 3334(0) 3138(0) 1625(0) 310(0) 5682(0) 5265(0) 5695(0) 4947(0) 1671(0) 855(0) 1751(0) 3374(0) Ueq 438(70) 438(70) 438(70) 830(60) 830(60) 830(60) 830(60) 830(60) 830(60) 830(60) 830(60) 438(70) 830(60) 830(60) 694(50) 694(50) 694(50) 694(50) 694(50) 694(50) 830(60) 830(60) 694(50) 694(50) 694(50) 694(50) 694(50) 694(50) Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Table SV. Complete list of bond distances (Å) angles (deg) of complex [Cu(tcq)Cl]CF3SO3·1.5H2O. Cu-N1 Cu-N2 Cu-N3 Cu-N4 Cu-Cl S-O1 S-O2 S-O3 S-C27 N1-C8 N1-C12 N2-C1 N2-C7 N3-C6 N3-C17 N4-C18 N4-C22 F1-C27 F2-C27 F3-C27 C1-C2 C1-C6 C2-C3 C3-C4 C4-C5 C5-C6 C7-C8 C8-C9 C9-C10 C10-C11 C11-C12 C11-C16 C12-C13 C13-C14 C14-C15 C15-C16 C17-C18 C18-C19 C19-C20 C20-C21 C21-C22 C21-C26 C22-C23 C23-C24 C24-C25 C25-C26 N1-Cu-N4 N1-Cu-Cl N2-Cu-N3 N2-Cu-N1 N2-Cu-N4 N2-Cu-Cl N3-Cu-N1 N3-Cu-N4 N3-Cu-Cl N4-Cu-Cl O1-S-O2 O1-S-O3 O1-S-C27 2.050(5) 2.013(5) 2.019(5) 2.269(5) 2.276(2) 1.401(6) 1.405(7) 1.401(6) 1.784(9) 1.343(7) 1.374(7) 1.471(8) 1.464(8) 1.487(8) 1.455(8) 1.320(8) 1.373(8) 1.328(11) 1.290(12) 1.290(11) 1.518(9) 1.504(8) 1.498(10) 1.522(11) 1.485(10) 1.526(9) 1.508(9) 1.373(9) 1.349(10) 1.415(8) 1.405(8) 1.421(9) 1.407(8) 1.361(9) 1.374(10) 1.347(9) 1.506(10) 1.408(9) 1.353(11) 1.388(10) 1.421(8) 1.405(10) 1.393(9) 1.361(10) 1.385(10) 1.341(12) 112.40(17) 97.28(14) 84.30(19) 81.06(19) 106.94(17) 151.49(14) 164.3(2) 77.36(18) 92.86(15) 100.02(13) 113.8(4) 113.2(4) 103.0(4) and Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 O2-S-O3 O2-S-C27 O3-S-C27 Cu-N1-C8 Cu-N1-C12 C8-N1-C12 Cu-N2-C1 Cu-N2-C7 C1-N2-C7 Cu-N3-C6 Cu-N3-C17 C6-N3-C17 Cu-N4-C18 Cu-N4-C22 C18-N4-C22 N2-C1-C2 N2-C1-C6 C2-C1-C6 C1-C2-C3 C2-C3-C4 C3-C4-C5 C4-C5-C6 N3-C6-C1 N3-C6-C5 C1-C6-C5 N2-C7-C8 N1-C8-C7 N1-C8-C9 C7-C8-C9 C8-C9-C10 C9-C10-C11 C10-C11-C12 C10-C11-C16 C12-C11-C16 N1-C12-C11 N1-C12-C13 C11-C12-C13 C12-C13-C14 C13-C14-C15 C14-C15-C16 C11-C16-C15 N3-C17-C18 N4-C18-C17 N4-C18-C19 C17-C18-C19 C18-C19-C20 C19-C20-C21 C20-C21-C22 C20-C21-C26 C22-C21-C26 N4-C22-C21 N4-C22-C23 C21-C22-C23 C22-C23-C24 C23-C24-C25 C24-C25-C26 C21-C26-C2 S-C27-F1 S-C27-F2 S-C27-F3 F1-C27-F2 F1-C27-F3 F2-C27-F3 117.3(4) 103.2(4) 104.1(4) 111.6(4) 129.2(4) 119.2(5) 109.2(3) 106.2(3) 116.1(5) 107.3(4) 110.6(4) 114.1(5) 108.8(4) 131.4(4) 118.5(5) 114.6(5) 106.5(5) 112.3(5) 112.3(6) 111.2(6) 111.7(6) 112.9(6) 107.4(5) 114.9(5) 110.7(5) 109.1(5) 115.4(5) 121.7(5) 122.9(6) 120.9(6) 119.5(6) 117.8(5) 123.6(6) 118.6(6) 120.8(5) 120.1(5) 119.1(5) 119.4(6) 122.2(6) 119.7(7) 120.9(6) 112.4(6) 117.1(5) 122.1(5) 120.8(6) 119.8(7) 120.5(7) 117.1(6) 124.1(7) 118.8(6) 121.9(5) 119.4(5) 118.6(6) 120.5(6) 120.7(6) 120.7(7) 120.6(7) 110.5(6) 113.4(7) 114.6(7) 104.4(8) 105.0(8) 108.2(8) Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Figure SI. Crystal structure of [Cu(tcq)Cl]CF3SO3·1.5H2O. Water molecules and CF3SO3 anion have been omitted for clarity C26 C20 C21 C25 C19 C24 C22 C18 C23 N4 C17 C14 N3 C13 Cl C15 C12 C16 C6 Cu N2 N1 C1 C11 C8 C10 C9 C7 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Figure SII. % of species vs pH with respect to total tcq for a 1×10-3 M solution of 1:1 tcq/Cu2+ Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Figure SIII. % of species vs pH with respect to total ccq for a 1×10-3 M solution of 1:1 ccq/Cu2+ Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Figure SIV. titration profile (emf vs ml of added base) for ligand tcq plus excess acid Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Figure SV. titration profile (emf vs ml of added base) for ligand ccq plus excess acid Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Figure SVI. titration profile (emf vs ml of added base) for ligand tcq plus one equivalent of CuII(CF3SO3)2 plus excess acid Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Figure SVII. titration profile (emf vs ml of added base) for ligand ccq plus one equivalent of CuII(CF3SO3)2 plus excess acid Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2002 Figure SVIII. emission spectra for Coumarine 343 on pH variation in dioxane/water 4:1