Chemical Reactions Introduction
advertisement

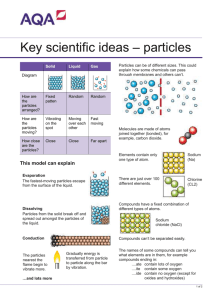
Section 3.1 Notes • Physical Change = a change in the state of matter • example = freezing liquid water into ice (still water, just in a different form) • Chemical change = changing an original substance into a completely new substance • example = sodium and chlorine reacting to make sodium chloride (table salt) • Chemical Reactions = a combination of different elements or compounds to create a new substance • represented through a chemical equation • example = Na + Cl2 --> NaCl2 • CH4 (methane) + 2O2 (oxygen) --> CO2 (carbon dioxide) + 2H2O (water) • reactants = present at the beginning of a reaction • methane and oxygen Products = present at the end of a reaction • • carbon dioxide and water Equation reads: methane plus oxygen yields carbon dioxide and water • • follow the direction of the reaction (arrow points to products) • At the molecular level, the bonds of the reactants must be broken and the bonds of the products have to be re-formed in order for a chemical reaction to occur Evidence of a Chemical Reaction • Color change = substances often change color during a chemical reaction • example = gray iron rusts to brown • Formation of a precipitate = forms when 2 chemicals in 2 liquids react to form a solid product • precipitate = solid that forms when chemicals in 2 liquids react • Formation of a gas = forms a gas product - seen as bubbling during the reaction • Temperature change = substances that involve a change in temperature • ranges from small changes in warmth to combustion (fire) reactions Rates of Reactions • 3 physical factors and 1 chemical factor that can greatly affect the rate (speed) of a chemical reaction Physical • Concentration = measures the number of particles present in a certain volume • increase the concentration to increase the rate of a reaction • increasing the number of particles present • Surface Area = number of particles in a solid that are fully exposed during a chemical reaction • increase the surface area to increase the rate of a reaction • increasing the number of particles exposed in a solid • Temperature = the energy (speed) in which molecules are moving - collisions of particles into each other • Increase the temperature to increase the rate of a reaction • increasing the speed of particles colliding with each other Chemical • Catalyst = a substance that increases the rate of a chemical reaction • add this to a chemical reaction to increase the rate of a reaction • A catalyst increases the rate of a chemical reaction, but is not used up (or dissolved) during a reaction Types of Chemical Reactions • Synthesis = one complex product is formed by the combination of simpler reactants • A + B --> AB • Decomposition = one complex reactant breaks down into simpler products • opposite of synthesis • AB --> A + B Combustion = one reactant is always oxygen, and the products are always carbon dioxide and • water • fire reaction • CH4 + 2O2 --> CO2 + 2H2O